An insulated container has 60 g of ice at −10∘C. 10 g steam at 100∘C, sourced from a boiler, is mixed to the ice inside the container. When thermal equilibrium was attained, the entire content of the container was liquid water at 0∘C. Calculate the percentage of steam (in terms of mass) that was condensed before it was fed to the container of ice. Specific heat and latent heat values are
Sice=0.5calg−1.∘C−1, Swater=1.0calg−1.∘C−1
Lfusion=80calg−1, Lvaporization=540calg−1
The correct Answer is:24%
Step by step video, text & image solution for An insulated container has 60 g of ice at -10^(@)C. 10 g steam at 100^(@)C, sourced from a boiler, is mixed to the ice inside the container. When thermal equilibrium was attained, the entire content of the container was liquid water at 0^(@)C. Calculate the percentage of steam (in terms of mass) that was condensed before it was fed to the container of ice. Specific heat and latent heat values are S_("ice") = 0.5 cal g^(-1) .^(@)C^(-1), S_("water") = 1.0 cal g^(-1) .^(@)C^(-1) L_("fusion") = 80 cal g^(-1), L_("vaporization") = 540 cal g^(-1) by Physics experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
Similar Questions
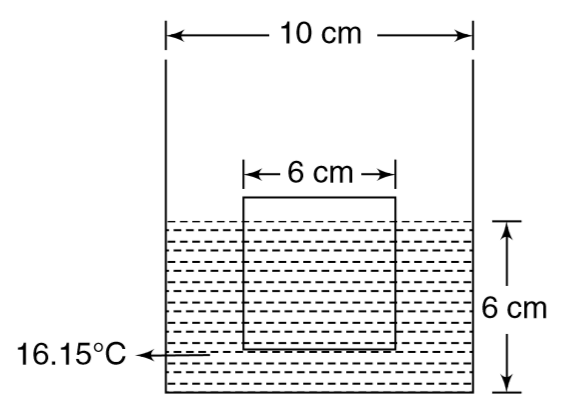
A container has a square cross-section of 10cm×10cm. A cubical ice block of side length 6 cm is floating in water in the container. Water level in the container is 6 cm high. The ice block is at a temperature of 0∘C and the water is at 16.15∘C. Assume that heat exchange take place between the ice block and water only. What length of ice block will remain submerged in water when the system reaches thermal equilibrium? Assume that the ice block maintains its cubical shape as it melts. Take - density of ice =0.9g/cc, density of water = 1.0g/cc
Specific heat capacity of water =1calg−1.∘C−1, Specific latent heat of fusion of ice =80calg−1
View SolutionA copper calorimeter has mass of 180 g and contains 450 g of water and 50 g of ice, all at 0∘C. Dry steam is passed into the calorimeter until a certain temperature (θ) is reached. The mass of the calorimeter and its contents at the end of the experiment increased by 25 g. Assume no heat loss to the surrounding and take specific heat capacities of water and copper to be 4200Jkg−1K−1 and 390Jkg−1K−1, respectively. Take specific latent heat of vaporization of water to be 3.36×105Jkg−1 and 2.26×106Jkg−1 respectively.
(a) Find the final temperature θ
(b) If steam enters into the system at a steady rate of 5g−1min, plot the variation of temperature of the system till final temperature θ is attained.
View SolutionIn an experiment mileage of a car was measured to be 24kmpl (Kilometer per liter of fuel consumed). After the experiment it was found that 4% of the fuel used during the experiment was leaked through a small hole in the tank. Calculate the actual mileage of the car after the tank was repaired.
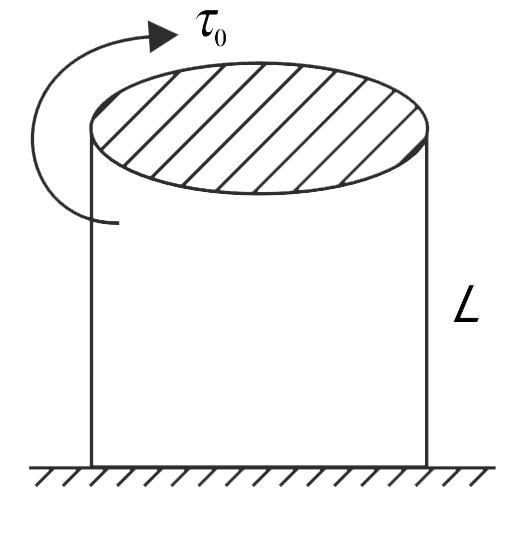
View SolutionA metal cylinder of length L and radius R is fixed rigidly to ground with its axis vertical. A twisting torque τ0 is applied along the circumference at the top of the cylinder. This causes an angular twist of θ0 (rad) in the top surface. Calculate the shear modulus of elasticity (η) of the material of the cylinder.
View Solutionm_(1) gram of ice at -10^(@)C and m_(2) gram of water at 50^(@)C are mixed in insulated container. If in equilibrium state we get only water at 0^(@)C then latent heat of ice is :
View Solution1 g of steam at 100^@C and an equal mass of ice at 0^@C are mixed. The temperature of the mixture in steady state will be (latent heat of steam =540 cal//g , latent heat of ice =80 cal//g ,specific heat of water =1 cal//g^@C )
View Solution20 g of steam at 100^@ C is passes into 100 g of ice at 0^@C . Find the resultant temperature if latent heat if steam is 540 cal//g ,latent heat of ice is 80 cal//g and specific heat of water is 1 cal//g^@C .
View SolutionWhat mass of steam at 100∘C must be mixed with 250g of ice at its melting point, in a thermally insulated container, to produce liquid water at 50∘C ?
View Solutionm1 gram of ice at −10∘C and m2 gram of water at 50∘C are mixed in insulated container. If in equilibrium state we get only water at 0∘C then latent heat of ice is :
View Solutionm1 gram of ice at −10∘C and m2 gram of water at 50∘C are mixed in insulated container. If in equilibrium state we get only water at 0∘C then latent heat of ice is :
View Solution20 g of steam at 100∘C is passed into 100 g of ice at 0∘C. Find the resultant temperature if specific latent heat of steam is 540 cal/g., specific latent heat of ice is 80 cal/g and specific heat of water is 1cal/g∘C.
View Solution60 g of ice at 0^(@)C is mixed with 60 g of steam at 100^(@)C . At thermal equilibrium, the mixture contains
View Solution20g of steam at 100∘C is passed into 100g of ice at 00C. Find the resultant temperature if specific latent heat of steam is 540 callg., specific latent heat of ice is 80 cal/g and specific heat of water is 1 cal/g0 C .
View Solution20g of steam at 100^(@) C is passed into 100g of ice at 0^(0) C. Find the resultant temperature if specific latent heat of steam is 540 callg., specific latent heat of ice is 80 cal/g and specific heat of water is 1 cal/ g^(0) C .
View SolutionHow much of ice will be converted into water at 0∘C if 10g of steam condenses into water at on a block of ice ? (latent heat of ice = 80cal/g, latent heat of steam = 540cal/g).
View Solution

