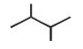
Which of the following azeotropic solution has the b.p. less than b.p. of the constituents A and B ?
A
CHCl3 and CH3COCH3
B
CS2 and CH3COCH3
C
CH3CH2OH and CH3COCH3
D
CH3 and CS2
Video Solution
Text Solution
The correct Answer is:C
Mixture of CH3CH2OH and CH_(3)COCH_(3) shows positive deviation from Raoult's law, so vapour pr. Increases and b.p decreases.
|
Topper's Solved these Questions
- SOLUTIONSBook:RESONANCEChapter:SOLUTIONSExercise:Advabced Level Problems (PART-2)
Explore 35 Videos
- SOLUTIONSBook:RESONANCEChapter:SOLUTIONSExercise:EXERCISE-3(PART-3)
Explore 30 Videos
- SOLUTION AND COLLIGATIVE PROPERTIESBook:RESONANCEChapter:SOLUTION AND COLLIGATIVE PROPERTIESExercise:PHYSICAL CHEMITRY (SOLUTION & COLLIGATIVE PROPERTIES)
Explore 52 Videos
- STEREOISOMERISMBook:RESONANCEChapter:STEREOISOMERISMExercise:EXERCISE (PART III : PRACTICE TEST-2 (IIT-JEE (ADVANCED PATTERN))
Explore 22 Videos
Similar Questions
Knowledge Check
Which of the following azeotropic solutions has the boiling point less than boiling point of the constituents A and B ?
ACHCI3andCH3COCH3BCS2andCH3COCH3CCH3CH2OHandCH3COCH3DCH3CHOandCS2Which of the following has lowest B.P
ACH3−CH2−CH2−NH2BCH3−CH∣NH2−CH3CC2H5−NH−CH3D(CH3)3NWhich of the following have maximum B.P
A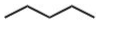 B
B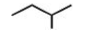 C
C D
D