If 2L of Cl2 gas and 2L of ClF3 gas react to form 6L of a pure gaseous compound at the same conditions of temperature and pressure, what is the molecular formula of the compound formed?
ClF
Cl2F2
Cl3F3
Cl2F5
The correct Answer is:A
Topper's Solved these Questions
Similar Questions
Does one gram mole of a gas occupy 22.4 L under all conditions of temperature and pressure ?
View Solution1.40L of an unknown gas requires 57 second to diffuse and the same volume of N2 gas takes 84 second to diffuse at the same temperature and pressure. What is the molecular mass of the unknown gas?
View Solution
Knowledge Check

L-form of the given compounds isA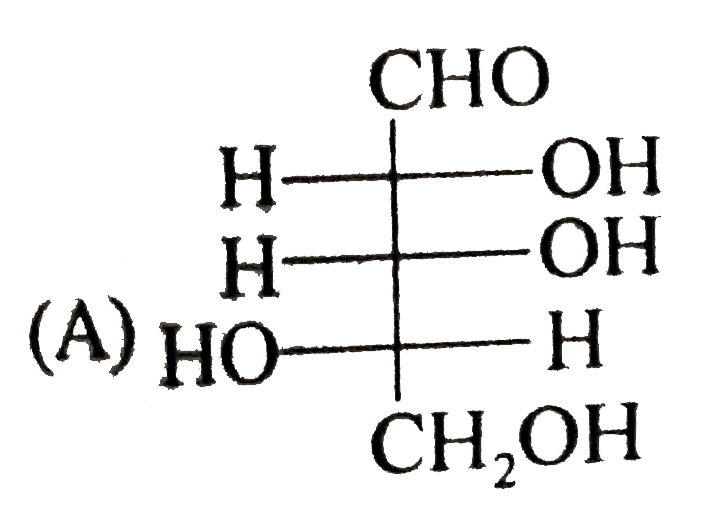 B
B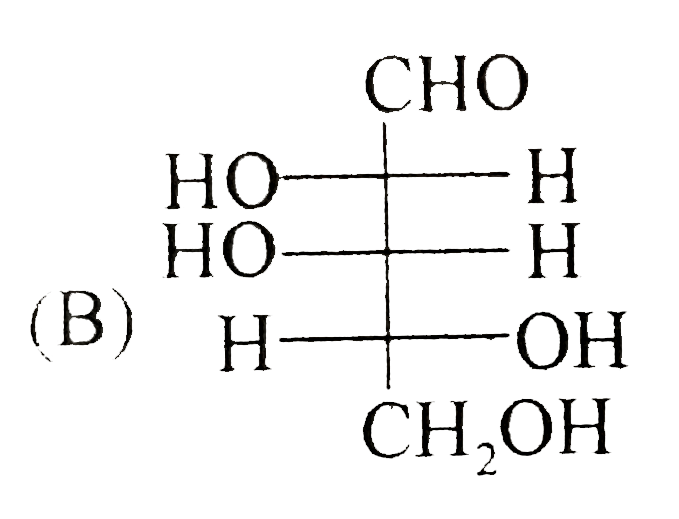 C
C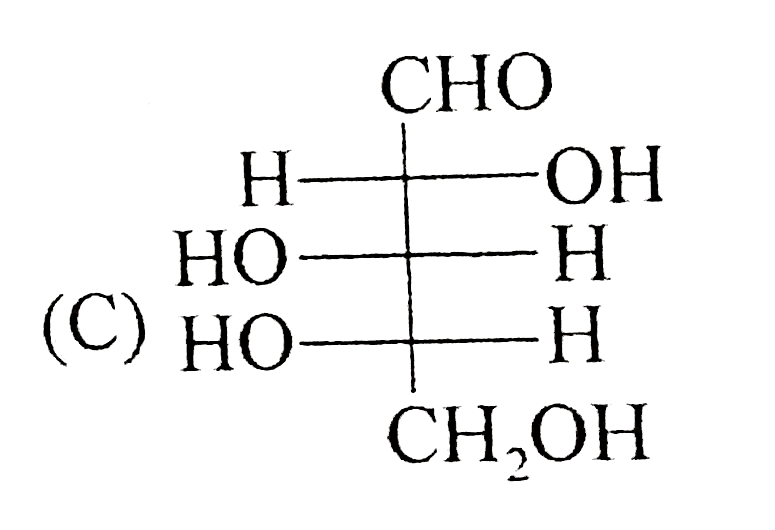 D
D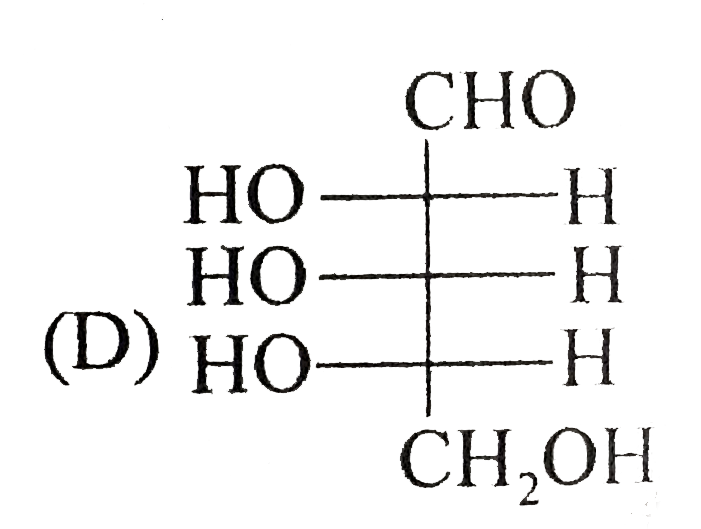
One volume of a gaseous organic compound of C, H and N on combustion produced 2 volumes of CO2, 3.5 volumes of H2O vapour and 0.5 volume of N2 under identical conditions of temperature and pressure. The molecular formula of the compound is
AC2H7NBC4H14N2CC2H7N2DC4H14N44.4 g of an unknown gas occupies 2.24 L of volume at standard temperature and pressure, The gas may be
ACarbon dioxideBCarbon monoxideCOxygenDSulphur dioxide
A mixture of one volume of gas and two volumes of oxygen, on explosion form two volumes of CO2 and one volume of N2 under the identical conditions of temperature and pressure. Find the formula of the gas.
View Solution5 L of methane gas at 2 atm pressure is compressed to 1.6 L at contant temperature. Calculate the final pressure.
View SolutionEthylene reacts with ozone gas to form the compound
View SolutionA gas has molecular formula (CH)n. If vapour density of the gas is 39, what should be the formula of the compound ?
View SolutionA pure gas that is 14.3% hydrogen and 85.7% carbon by mass has a density of 2.5gL−1 at 0∘C and 1 atm pressure. What is the molecular formula of the gas :
View Solution

