Explain the following
(i)H3PO3 is a dibasic acid.
(ii) Phosphine has lower boiling point than ammonia.
Video Solution
|
Answer
Step by step video & image solution for Explain the following (i)H_3 PO_3 is a dibasic acid. (ii) Phosphine has lower boiling point than ammonia. by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
Similar Questions
Knowledge Check
In which case second has lower boiling point than first ?
A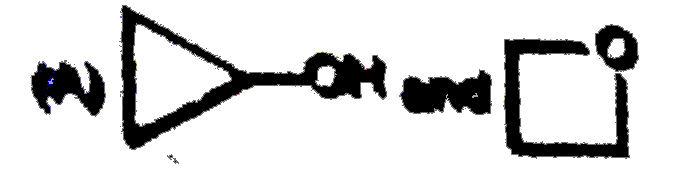 B
B C
C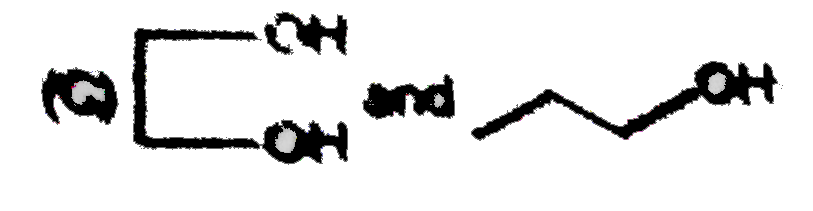 D
D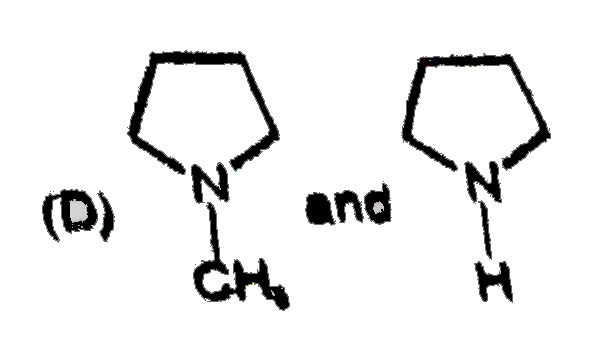
PH3 has lower boiling point that NH3. Why ?
View SolutionPH3 has lower boiling point that NH3. Why ?
View SolutionExplain why A has lower point than B ?
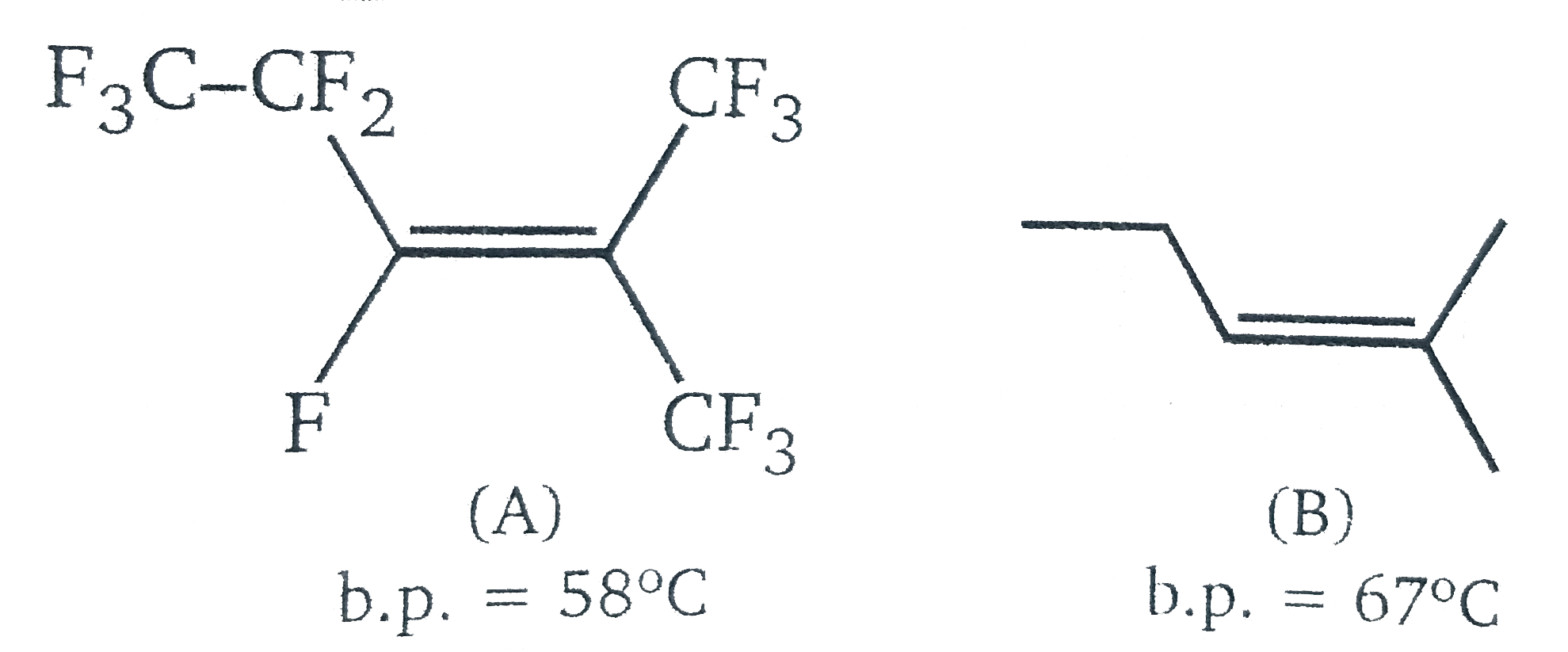
View SolutionPH3 has lower boiling point that NH3. Why ?
View SolutionGive reasons for the following.
a. LiCI is more covalent than KCI.
b. LiI has lower melting point than LiF.
c. During electrolysis of molten sodium chloride, calcium chloride and potassium fluoride are added.
View SolutionH3PO3 is a diprotic acid. Justify.
View SolutionWhy does PH3 have lower boiling point than NH3 ?
View Solution
