For the reaction PCl5(g)⇔PCl3(g)+Cl2(g) the forward reaction at constant temperature is favoured by
introducing an inert gas at constant volume
introducing chlorine gas at constant volume
increasing the volume of the container
introducing PCl5 at constant volume
The correct Answer is:C, D
Step by step video, text & image solution for For the reaction PCl_(5)(g)hArrPCl_(3)(g)+Cl_(2)(g) the forward reaction at constant temperature is favoured by by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- CHEMICAL AND IONIC EQUILIBRIUMBOOK - IIT-JEE PREVIOUS YEAR (CHEMISTRY)CHAPTER - CHEMICAL AND IONIC EQUILIBRIUMEXERCISE - OBJECTIVE_TYPE
3 Videos
- CHEMICAL AND IONIC EQUILIBRIUMBOOK - IIT-JEE PREVIOUS YEAR (CHEMISTRY)CHAPTER - CHEMICAL AND IONIC EQUILIBRIUMEXERCISE - COMPREHENSION_TYPE
3 Videos
- CARBOXYLIC ACID AND THEIR DERIVATIVESBOOK - IIT-JEE PREVIOUS YEAR (CHEMISTRY)CHAPTER - CARBOXYLIC ACID AND THEIR DERIVATIVESEXERCISE - CHAPTER TEST
3 Videos
- CHEMICAL BONDINGBOOK - IIT-JEE PREVIOUS YEAR (CHEMISTRY)CHAPTER - CHEMICAL BONDINGEXERCISE - MATCH THE COLUMN
1 Video
Similar Questions
Which of the following statement(s) is (are) correct?
View SolutionTo determine molar solubility of an unknown metal hydroxide, M(OH)3 in 0.00101MNaOH, Jane adds 0.250g of M(OH)3 solid to 200mL of a 0.00101MNaOH solution and stirred for a long time.A cloudy solution is formed. The undissolved hydroxide is separated from the solution by filtratin 25.00mL of filtrate is pipetted into a flask 30mL of water is added adn then the solution is titrated with 0.00444MHCl solution 13mL of HCl is used for this titration.
The molar concentration of hydroxide ion in filtrate solution is
View SolutionEthyl acaetate undragoes the followinfg sequence of reactions
treatment with excess phenylmagnesium bromide in ether
heating with cono.H3PO4
treatment B2H6 in ether, followed by alkaline H2O2
treatment with jones' regent (CrO3in aqueous acid+acetone)
View SolutionMatch the reactions in Column I with nature of the reactions/type of the products in Column II
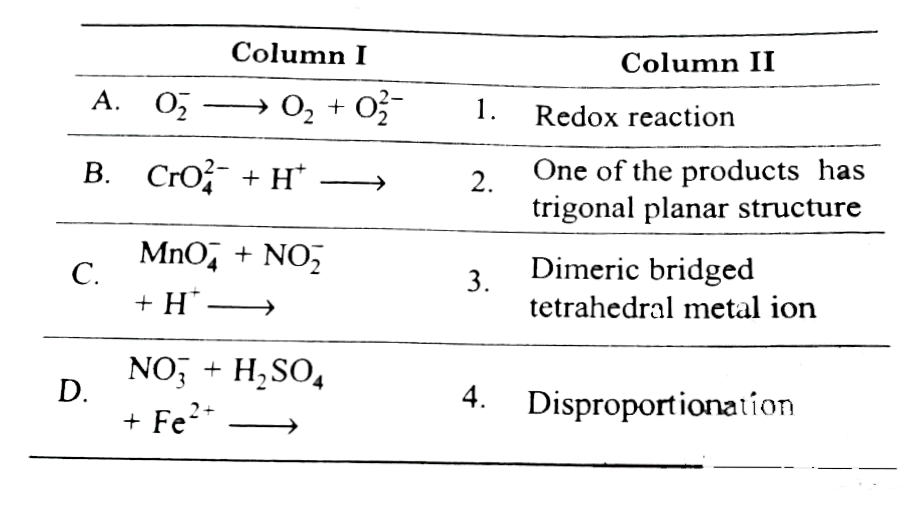
View SolutionFor the reaction PCl_(5)(g) hArr PCl_(3)(g)+Cl__(2)(g) The forward reaction at constant temperature is favoured by
View SolutionFor the reaction, PCl_(5(g))hArrPCl_(3(g))+Cl_(2(g)) , the forward reaction at constant temperature is favoured by:
View SolutionFor the reaction PCl5(g)⇔PCl3(g)+Cl2(g), the forward reaction at constant temperature favorrd by :
View SolutionFor the reaction PCl5(g)⇔PCl3(g)+Cl2(g) the forward reaction at constant temeprature is favoured by
View SolutionFor the reaction Pcl_(5(g))hArrPCl_(3(g))+Cl_(2(g))
View SolutionFor the reaction, PCl_(5)(g)to PCl_(3)(g)+Cl_(2)(g), The forward reaction at constant temperature is favoured by
View SolutionFor the reaction PCl _(5) (g) hArr PCl_(3)(g) + Cl_(2) (g), the forward reaction at constant temperature is favoured by
View SolutionAt a given temperature the equilibrium constant for the reaction of PCl_(5)hArrPCl_(3)+Cl_(2) is 2.4xx10^(-3) . At the same temperature, the equilibrium constant for the reaction PCl_(3)(g)+Cl_(2)(g)hArrPCl_(5)(g) is :
View SolutionFor the reaction PCl_(5) (g) hArr PCl_(3) (g) + Cl_(2) (g) the forward reaction at constant temperature is favoured by …………
View SolutionConsider the following reaction, PCl_(5) (g) hArr PCl_(3) (g) + Cl_(2)(g) the forward reaction at constant temperature is favoured by
View SolutionFor the reaction PCl5(g)⇔PCl3(g)+Cl2(g) the forward reaction at constant temperature is favoured by
View Solution

