Lucas test is used for distinguishing primary, secondary and tertiary alchols as:
ROH+Conc.HClZnCl2−−−→RCl(white turbidity)+H2O
The correct statement regarding the above test is/are
ROH behaves as a base
Greater the value of pKa of alcohol, greater the reactivity with HCl and thus, sooner the formation of white turbidity.
Alcohols that reacts faster with Na metal, will give turbidity at fastest rate.
Alcohols that do not change the orange colour of acidic dichromate solution produces immediate turbidity in this test.
The correct Answer is:a,b,d
Topper's Solved these Questions
Similar Questions
Knowledge Check
2Al+N2→XH2O−−→Y+Z↑⏐⏐
Correct statement regarding YAY is soluble in H2OBY is insoluble in H2OCY is only basic and does not show acidic behaviourDM.W.of'Y' is 17Lucas test is used to make distinguation between 1∘,2∘and3∘ alcohols.
ROH+HClconc.anydrous ZnCl2−−−−−−−−→RCl↓whiteturvidity+H2O
This shown that -AROH behavs as a baseBgreater the value of pKa (alcohols), greater the reactivity with conc. HCl and thus sonner the formation of white tarbidityCboth of the above are correctDnone of the above is correctH2O↔H2O(vap.)point out the correct statement for above reaction
ARate of condensation=rate of sublimationBRate of sublimation=rate of evaporationCRare of evaporation=rate of condensationDnone of the above
Lucas test is usedto make distinction between1∘2∘and3∘alcohols
ROH+Hclconc.AnhydrousZnCl2−−−−−−−−−→RCI↓White turbidity+H2O
This shows that :
View SolutionLucas test is used to make distinguation between 1∘,2∘and3∘ alcohols.
ROH+HClconc.anydrous ZnCl2−−−−−−−−→RCl↓whiteturvidity+H2O
This shown that -
View SolutionWhich of the following chemical tests can be used to distinguish primary . Secondary and tertiary alcohol from each other ?
View SolutionAlcohols are acidic in nature because hydrogen is attached to oxygen which is an electronegative element. Alcohol is weaker acid than water because OH− ion is more stable than R−O− ion. R−O− is less stable, due to +I effect of the alkyl group. Acidity of alcohols increases with increase in the stability of acid anion.
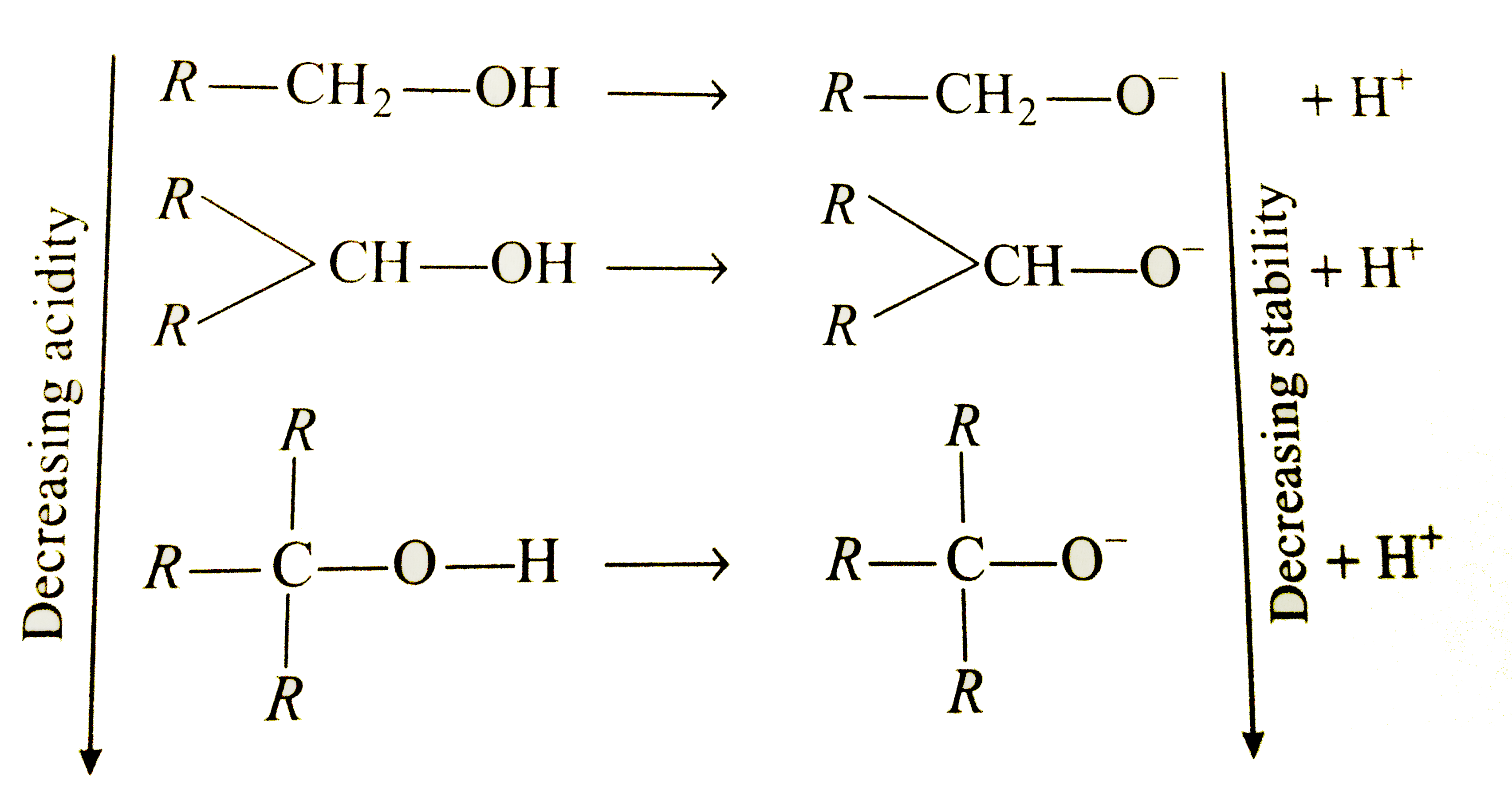
Some alcohol like CH3−OH,CF3−CH2−OH,(CF3)3C−OH ae more acidic than water.
Lucas test of alcohols involves following reaction :
R−OH+HClconc.anhydrous−−−−−−−−→ZnCl2RClwhite turbidity+H2O
Select the correct statements for the Lucas test.
View SolutionWhat is the correct order of reactivity of alcohols in the following reaction ?
R−OH+HClZnCl2−−−→R−Cl+H2O
View Solution

