p-amino-N, N-dimethyl laniline is added to a strongly acidci solution of x. The resulting solution is treated with a few drops of aqueous solution of Y to yield blue colouration due to the formation of methylene, blue. Treatment of the aqueous solution of Y with the reagent potassium hexaxyanoferrate (II) leads to the formation of an intense blue precipitate. The precipitate of the solution of Y with the solution of potassium hexacyanoferrate (III) leads to a brown colouration due to the formation of Z.
Mg2[Fe(CN)6]
Fe[Fe(CN)6]
Fe4[Fe(CN)6]3
K2Zn3[Fe(CN)6]2
The correct Answer is:B
Topper's Solved these Questions
- QUALITATIVE ANALYSISBOOK - IIT-JEE PREVIOUS YEAR (CHEMISTRY)CHAPTER - QUALITATIVE ANALYSISEXERCISE - Fill in the blanks
1 Video
- QUALITATIVE ANALYSISBOOK - IIT-JEE PREVIOUS YEAR (CHEMISTRY)CHAPTER - QUALITATIVE ANALYSISEXERCISE - True/False
1 Video
- QUALITATIVE ANALYSISBOOK - IIT-JEE PREVIOUS YEAR (CHEMISTRY)CHAPTER - QUALITATIVE ANALYSISEXERCISE - Objective Question I
3 Videos
- PERIODIC CLASSIFICATION AND PERIODIC PROPERTIESBOOK - IIT-JEE PREVIOUS YEAR (CHEMISTRY)CHAPTER - PERIODIC CLASSIFICATION AND PERIODIC PROPERTIESEXERCISE - COMPREHENSION_TYPE
3 Videos
- S BLOCK ELEMENTSBOOK - IIT-JEE PREVIOUS YEAR (CHEMISTRY)CHAPTER - S BLOCK ELEMENTSEXERCISE - True/False
1 Video
Similar Questions
If metal ions of groups III are precipitated by NH4Cl and NH4OH without prior oxidation by conc. HNO3....... is not completely precipitated.
View SolutionAddition of ammonium chloride to a solution containing ferric and magnesium ions is essential for selective precipitation of ferric hydroxide by aqueous ammonia.
View SolutionUpon treatment with ammoniacal H2S, the metal ion that precipitates as a sulphide is
View SolutionLet's consider a hypothetical planet pseudo Earth which is similar to our earth in several aspects. The similarities are
On pseudo earth:
(i) There are same number of elements as on our earth and they are known by the same name.
(ii) Pauli's exclusion principal, Hund's Rule and Aufbau principle are known to the people of pseudo earth in the same manner as we know on our earth.
(iii) They classify elements as representative, transition and inner- transition elements in the same manner as we classify on our earth.
However, there is one basic difference in understanding the electrons, spin on these two earths. On our earth the electron can have only two spin directions. clock wise (1) and anti-clockwise (2), while on pseudo earth there is an additional possible value of spin quantum number called neutral spin (3) in which electron is believed to be fluctuating harmonically between clockwise and anti-clockwise directions, about its axis. Answer the following three question based on the above information.
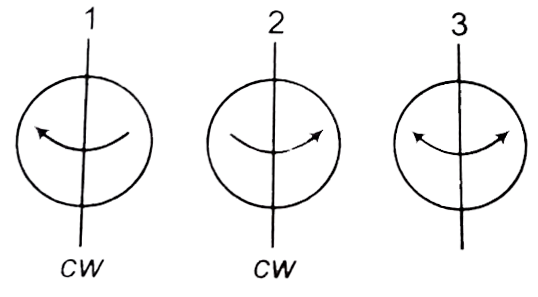
The first noble gas on pseudo earth would be
View SolutionMgCl2.6H2O on heating gives anhydrous MgCl2
View Solutionp-Amino-N,N-dimethylaniline is added to a strongly acidic solution of X. The resulting solution is treated with a few drops of aqueous solution of Y to yield blue colouration due to the formation of methylene blue. Treatment of aqueous solution of Y with reagent potassium hexacyanoferrate (II) leads to the formation of an intense blue precipitate. The precipitate dissolves on excess addition of the reagent. Similarly, the treatment of the solution of Y with the solution of potassium hexacyanoferrate (III) leads to a brown colouration due to the formation of Z. Q. Compound X is
View Solutionp-Amino-N,N-dimethylaniline is added to a strongly acidic solution of X. The resulting solution is treated with a few drops of aqueous solution of Y to yield blue colouration due to the formation of methylene blue. Treatment of aqueous solution of Y with reagent potassium hexacyanoferrate (II) leads to the formation of an intense blue precipitate. The precipitate dissolves on excess addition of the reagent. Similarly, the treatment of the solution of Y with the solution of potassium hexacyanoferrate (III) leads to a brown colouration due to the formation of Z. Q. Compound Y is
View Solutionp-Amino-N,N-dimethylaniline is added to a strongly acidic solution of X. The resulting solution is treated with a few drops of aqueous solution of Y to yield blue colouration due to the formation of methylene blue. Treatment of aqueous solution of Y with reagent potassium hexacyanoferrate (II) leads to the formation of an intense blue precipitate. The precipitate dissolves on excess addition of the reagent. Similarly, the treatment of the solution of Y with the solution of potassium hexacyanoferrate (III) leads to a brown colouration due to the formation of Z. Q. Compound Z is
View Solutionp-amino-N, N-dimethyl laniline is added to a strongly acidci solution of x. The resulting solution is treated with a few drops of aqueous solution of Y to yield blue colouration due to the formation of methylene, blue. Treatment of the aqueous solution of Y with the reagent potassium hexaxyanoferrate (II) leads to the formation of an intense blue precipitate. The precipitate of the solution of Y with the solution of potassium hexacyanoferrate (III) leads to a brown colouration due to the formation of Z.
The compound X, is
View Solutionp-amino-N, N-dimethyl laniline is added to a strongly acidci solution of x. The resulting solution is treated with a few drops of aqueous solution of Y to yield blue colouration due to the formation of methylene, blue. Treatment of the aqueous solution of Y with the reagent potassium hexaxyanoferrate (II) leads to the formation of an intense blue precipitate. The precipitate of the solution of Y with the solution of potassium hexacyanoferrate (III) leads to a brown colouration due to the formation of Z. The compound Y, is
View Solutionp-Amino-N,N-dimethylaniline is added to a strongly acidic solution of X. the resulting solution is treated with a few drops of aqueous solution of Y to yield blue colouration due to the formation of methylene blue. Treatment of the aqueous solution of Y with the reagent potassium hexacyanoferrate (II) leads to the formation of an intense blue precipitate. The precipitate dissolves on excess addition of the reagent. Similarly, treatment of the solution of Y with the solution of potassium hexacyanoferrate (III) leads to a brown colouration due to the formation of Z.
The compound X is:
View Solutionp-Amino-N,N-dimethylaniline is added to a strongly acidic solution of X. the resulting solution is treated with a few drops of aqueous solution of Y to yield blue colouration due to the formation of methylene blue. Treatment of the aqueous solution of Y with the reagent potassium hexacyanoferrate (II) leads to the formation of an intense blue precipitate. The precipitate dissolves on excess addition of the reagent. Similarly, treatment of the solution of Y with the solution of potassium hexacyanoferrate (III) leads to a brown colouration due to the formation of Z.
The compound Y is:
View Solutionp-Amino-N,N-dimethylaniline is added to a strongly acidic solution of X. the resulting solution is treated with a few drops of aqueous solution of Y to yield blue colouration due to the formation of methylene blue. Treatment of the aqueous solution of Y with the reagent potassium hexacyanoferrate (II) leads to the formation of an intense blue precipitate. The precipitate dissolves on excess addition of the reagent. Similarly, treatment of the solution of Y with the solution of potassium hexacyanoferrate (III) leads to a brown colouration due to the formation of Z.
The compound Z is:
View Solutionp-Amino-N,N-dimethylaniline is added to a strongly acidic solution of X.The resulting solution is treated with few drops of aqueous solution of Y to yield blue colouration due to the formation of methylene blue.Treatment of the aqueous solution of Y with the reagent potassium hexacyanoferrate(II) leads to the formation of an intension blue precipitate.The precipitate dissolves on excess addition of the reagent.Similarly, treatment of the solution of Y with the solution of potassium hexacyanoferrate(III) leads to a brown coloration due to the formation of Z.
The compound X is:
View Solutionp -Amino- N,N -dimethylaniline is added to a strongly acidic solution of X .The resulting solution is treated with few drops of aqueous solution of Y to yield blue colouration due to the formation of methylene blue.Treatment of the aqueous solution of Y with the reagent potassium hexacyanoferrate (II) leads to the formation of an intension blue precipitate.The precipitate dissolves on excess addition of the reagent.Similarly, treatment of the solution of Y with the solution of potassium hexacyanoferrate (III) leads to a brown coloration due to the formation of Z . The compound Y is :
View Solution

