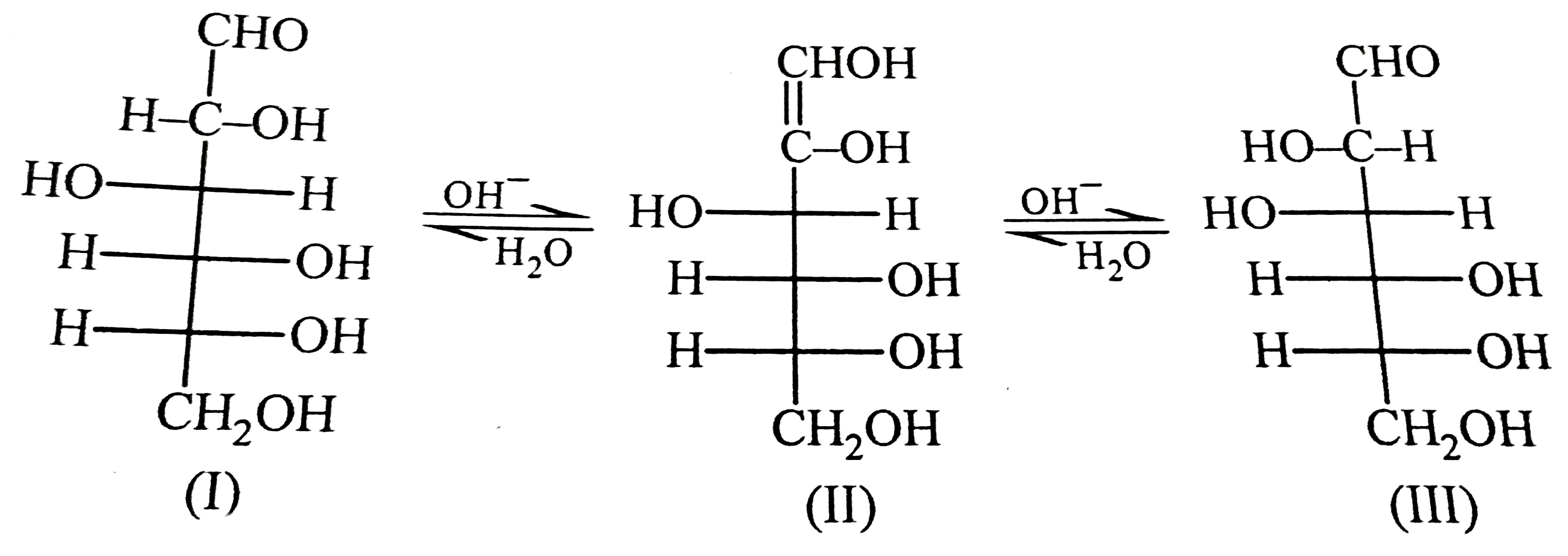
Consider the given process.
And identify the incorrect statement
A
Configuration at C−2 is lost on enolisation
B
I and III are epimers
C
Proton transfer from water to C−1 converts ene diol to an aldose
D
D-glucose can isomerise to D-fructose through enol intermediate.
Text Solution
The correct Answer is:C
D-glucose can isomerise to D-fructose through enol intermediate
|
Topper's Solved these Questions
- GENERAL ORGANIC CHEMISTRYBOOK - BANSALCHAPTER - GENERAL ORGANIC CHEMISTRYEXERCISE - Exercise 2
39 Videos
- GENERAL ORGANIC CHEMISTRYBOOK - BANSALCHAPTER - GENERAL ORGANIC CHEMISTRYEXERCISE - Exercise 3
9 Videos
- GENERAL ORGANIC CHEMISTRYBOOK - BANSALCHAPTER - GENERAL ORGANIC CHEMISTRYEXERCISE - Exercise 3
9 Videos
- ATOMIC STRUCTUREBOOK - BANSALCHAPTER - ATOMIC STRUCTUREEXERCISE - Exercise.3
35 Videos
- HYDROCARBONSBOOK - BANSALCHAPTER - HYDROCARBONSEXERCISE - Exercise 3
12 Videos
Similar Questions
Knowledge Check
Identify the incorrect statements.
AAt Boyle's temperature, there exists a pressure where compressibility of a real gas is more than that of ideal gas.BThe parent and daughter nuclei in a α-decay are isodiaphers.CThe magnetic moment of Cr+1 is more than that of Cr.DThe equivalent weight of Na2CO3 in its titration with HCl is 106 when phenolphthalien is used as an indicator.Identify the incorrect statements .
Arusting of iron in the presence of the air and moisture, is a slow reactionBinversio of a cane sugar occurs at a moderate rateChydrolysis of starch is a fast reactionDionic reactions are the examples of fast reactionsIdentify the incorrect statement(s):
ADensity of Mg is less than CaBThe atomic radius of Mg is greater than that of CaCMg alloys are used in the contruction of air craftsDMg is used as a reducing agent

