For zero order reaction :
A+2B→Product
If initial concentration of A is 2a and rate law.
−dBdt=K[A]o[B]o
then t1/2 of A will be
a2K
2aK
aK
a3K
The correct Answer is:A
Ecell=2.303RTF×log.[Ag+]Cathode[Ag+]anode
6×10−2=0.06log0.01[Ag+]anode
[Ag+]anode=10−3M
Ksp=∣∣Ag+∣∣.2∣∣CrO−24∣∣=10−6×0.01=10−8
10−8=S2×1 ⇒ S=10−4
104×S=104×10−4=0or1
Topper's Solved these Questions
Similar Questions
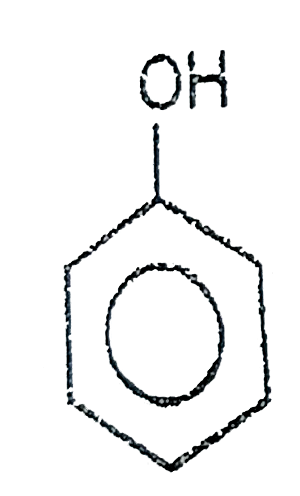 , CO2/NaOH−−−−−−−−−→H+(A)Acetic Anhydride−−−−−−−−−→Aspirin(B)
, CO2/NaOH−−−−−−−−−→H+(A)Acetic Anhydride−−−−−−−−−→Aspirin(B)
Product B is
View SolutionThe gaseous reaction
2A(g)+nB(g)⇔mC(g) is represented by following curves.
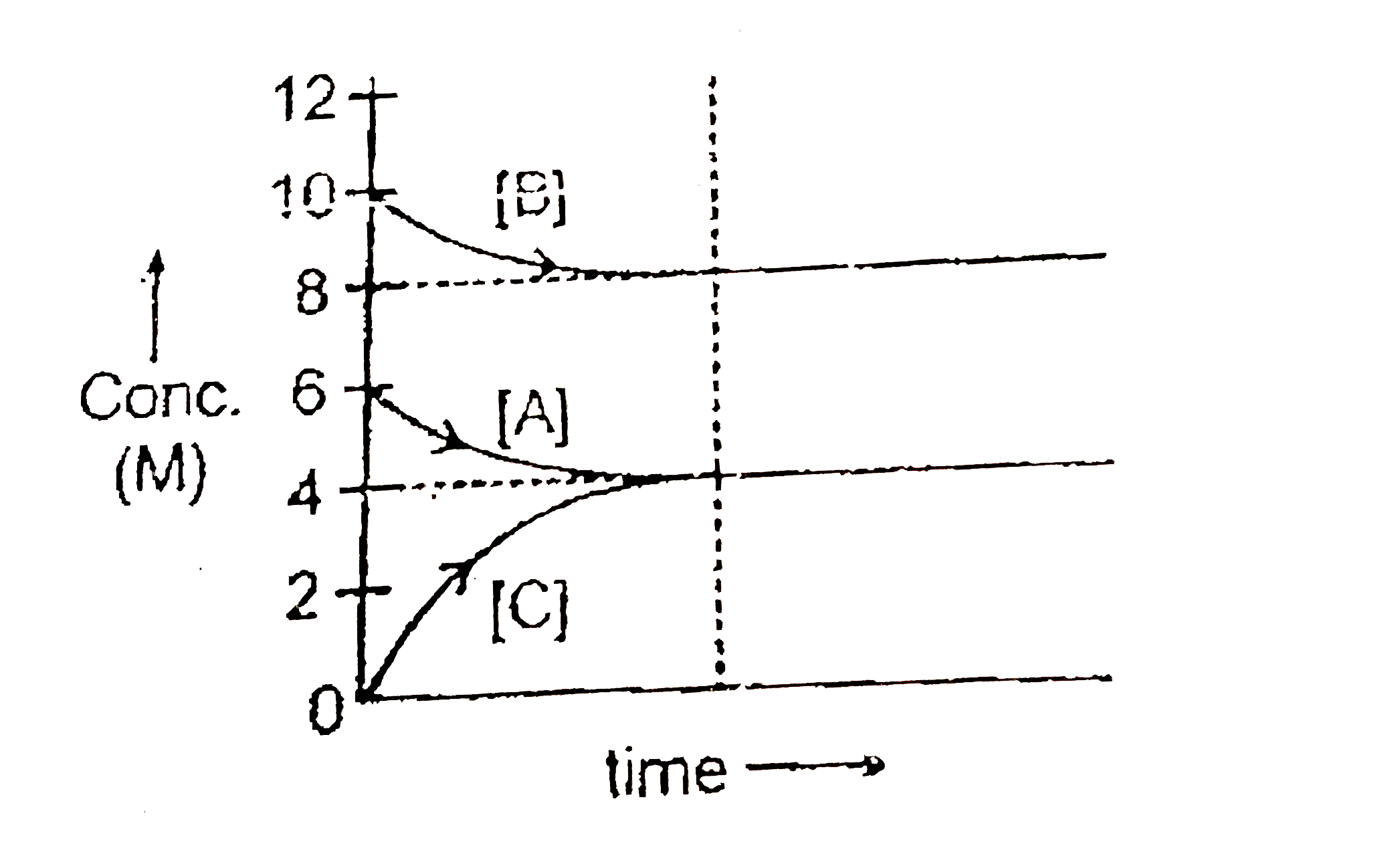
What is the value of m+n?
View SolutionThe rate law of the reaction A+2B→ Product is given by d(Product)dt=k[A]2[B]. A is taken in large excess, the order of the reqaction will be
View Solutionअभिक्रिया A+2B→ उत्पाद का वेग नियम निम्नलिखित है
d[P]dt=K[A]2[B]
यदि A आधिक्य में लिया जाए तो अभिक्रिया की कोटि होगी
View SolutionThe initial rate, -(d[A])/(dt) at t=o was found to be 2.6xx10^(2)" mol "L^(-1)s^(_1) for the reaction A+2Brarr"products" The initial rate, -(d[B])/(dt), at t=o is
View SolutionFor the zero order reaction, A → Products, with rate constant k, half-life period is given by t1/2 =………………… .
View SolutionFor a reaction 2A + B rarr product, rate law is -(d[A])/(dt)=k[A] . At a time when t=(1)/(k) , concentration of the reactant is " (C_(0)="initial concentration")
View SolutionFor a zero order reaction , the correct expression for rate constant (k) at half-life time (t_(1//2)) is R_0 = initial concentration of reactant )
View SolutionFor the reaction A B, the rate law expression is −d[A]dt=k[A]1/2. If initial concentration of [A] is [A]0, then
View SolutionFor zero order reaction , t_(1//2) will be ( A_0 is the initial concentration , K is rate constant)
View SolutionA : The rate constant of zero order reaction is equal to rate of reaction. R : t_(1//2) for zero order reaction is directly proportional to initial concentration.
View SolutionIn a first order reaction A to B , if k is rate constant and initial concentration o f the reactant A is 0.5 M then the half-life is
View SolutionFor the reaction 2A+B to product , (d[A])/(dt)=K[A]^(2)[B] . What will be rate equation for -(d[B])/(dt) ?
View SolutionThe rate law of the reaction A + 2Brarr Product is given by (d[P])/dt=K[A]^2[B] . If A is take in large excess the order of the reaction will be:
View SolutionThe rate law expression for a hypothetic reaction:
2A+3B→2C is
dxdt=k[A][B]2
The order of the reaction is
View Solution

