B2H6+2L→2BH3L(Lewis base)
L would be :
A
NH3
B
CH3NH2
C
(CH3)2NH
D
(CH3)3N
Text Solution
The correct Answer is:D
N/A
|
Similar Questions
Knowledge Check
The product (z) in the following sequence of reaction is
C6H6+CH2=CHCH2ClAlCl3−−→(X)(i) BH3/THF−−−−−−−−−−→(ii) H2O2,OH(j)HF−−→heatC9H10(z)A B
B C
C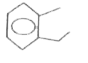 Dnone of the above
Dnone of the aboveB2H6 or (BH3)2 is an example of :
Aelectron-rich hydrideBmetallic hydrideCsaline hydrideDmolecular hydrideIn the following reaction
2X+B2H6→[BH2(X)2]+[BH4]−
then X will be : -ANH3BCH3NH2CNaHDAll of these
In the following reaction.
2X+B2H6→[BH2(X)2]⊕[BH4]Ө
The amine (s)x is /are.
View SolutionBCl3 does not exist as dimer but BH3 exist as dimer (B2H6) because :-
View SolutionBCl3 does not exist as dimer but BH3 exist as dimer (B2H6) because :-
View SolutionIn the following reaction.
2X+B2H6→[BH2(X)2]⊕[BH4]Ө
The amine (s)x is /are.
View SolutionBi) BH3/THF−−−−−−−−→ii) H2O2/OH
 A and Bare respectively
A and Bare respectively
View Solution

