Select true statement(s):
A
Resonance affect bond order
B
E-2-chlorobut-2-ene & cis-2-chlorobut-2-ene are identical compounds
C
H−C∣∣O−OH is more acidic than .−O−C∣∣O−C∣∣O−OH
D
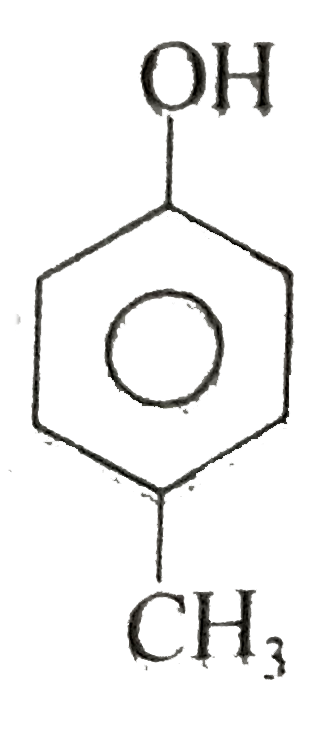
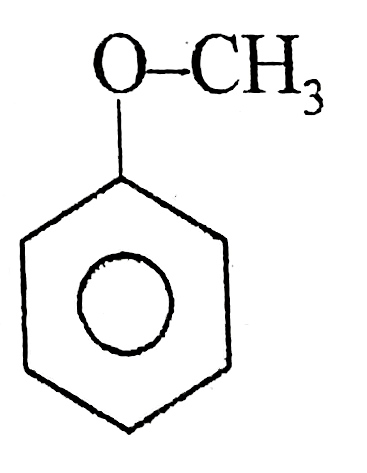 Boiling point of is greater than boiling point of
Boiling point of is greater than boiling point of
Video Solution
Text Solution
The correct Answer is:A, B, C, D
(A). Bond order=Total number of bondsTotal number of resonanting structures
(B). 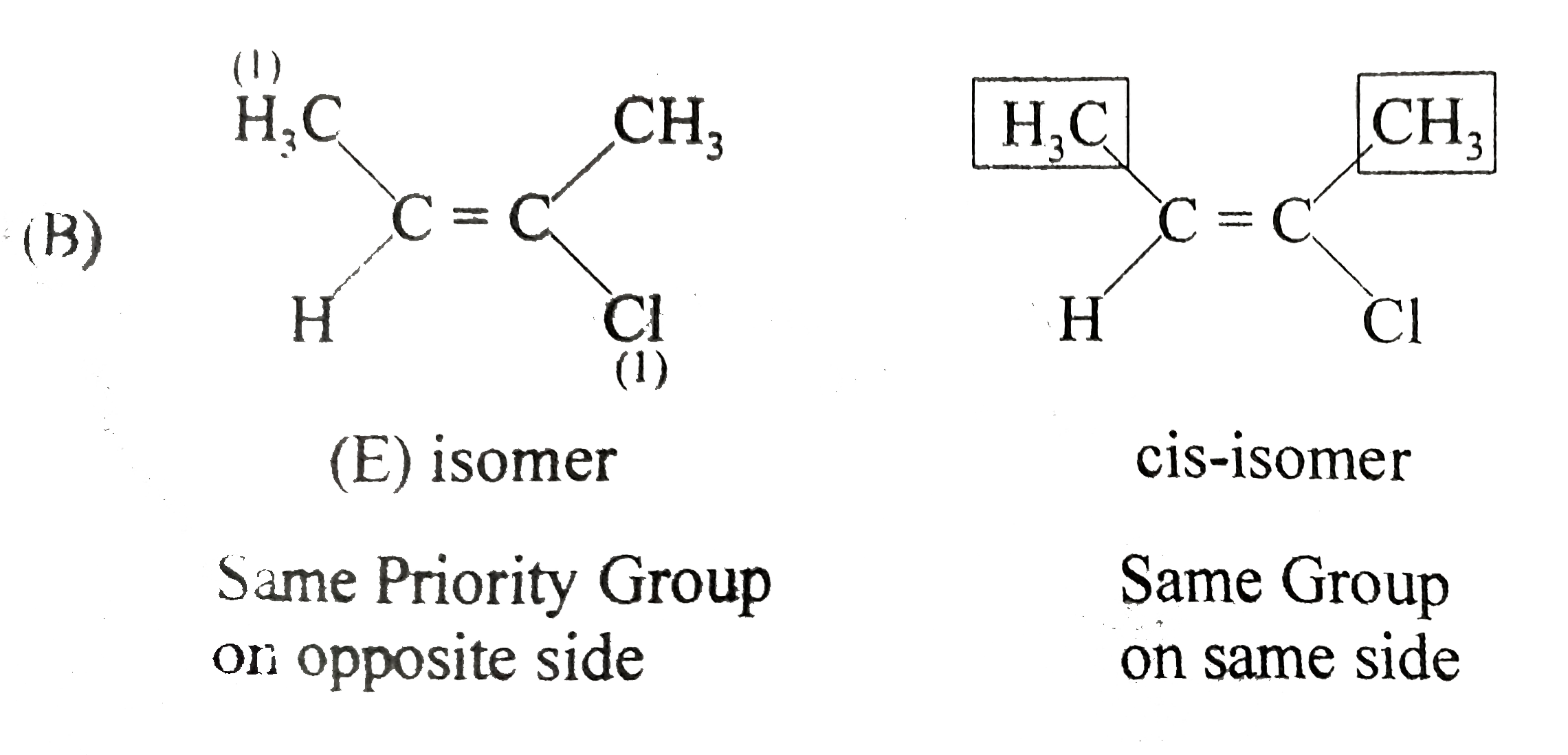
(C). 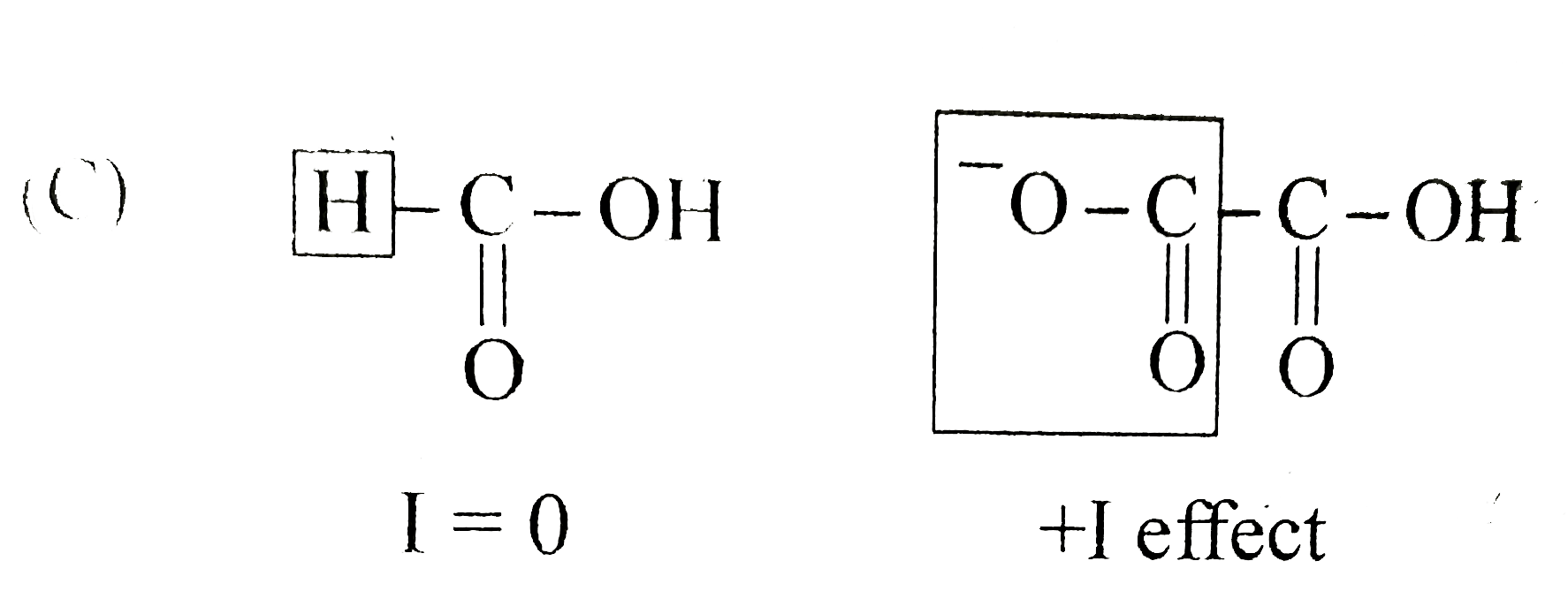
Acidic strength α−I+I
(D). Boiling point of alcohol > ether due to hydrogen bonding
|
Similar Questions
Knowledge Check
Select the true statements (s)
ABelow 710∘C, C is better reducing agent than COBBelow 710∘C, CO is better reducing agent than CCBelow 710∘C, CO is an oxidising agentDBelow 710∘C,CO2 is reducing agentSelect the true statement(s) among the following :
APure overlapping of two dxy orbitals along x-axis results in the formation of π-bondBNO+2>NO−3>NO−2 is the correct order of bond angle as well as N-O bond orderCNF3<NCl3<NBr3<NI3 is the correct order of Lewis basic character as well as bond angleDHF>HCl>HBr>HI is the correct order of dipole moment as well as boiling pointSelect the true statement
ABelow 710∘C, C is better reducing agent than COBBelow 710∘C, CO is better reducing agent than CCBelow 710∘C, CO is an oxidising agentDBelow 710∘C,CO2 is reducing agent
Select the true statement(s) for AUG. A. It codes for an amino acid methionine B. It is an initiation codon C. It is a non-degenerate codon
View SolutionSelect the true statement
View SolutionSelect the true statements:
View SolutionSelect the true statement -
View Solution
Select the true statement
View Solution
