Which of the following pairs has higher resonance energy:
(a). CH3COOH&CH3COONa
(b). CH2=CH−ΘO&CH2=CH−OH
The correct Answer is:(a)II,(b)I
(a). CH_(3)-overset(O)overset(||)(C)-overset(ɵ)(O)overset(o+)(N)a lone pair easily available for resonance.
(b). In 1st part 1.p. easily available.
Similar Questions
Which of the following pairs has less resonance energy:

and CH2=CH−CH=CH2
View SolutionWhich of the following pairs has less resonance energy:

Ө" and "CH_(2) = CH - CH_(2)^(-)
View SolutionWhich of the following pairs has higher resonance energy:
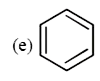 and CH2=CH−CH=CH−CH=CH2
and CH2=CH−CH=CH−CH=CH2
View SolutionWhich of the following pairs has less resonance energy:
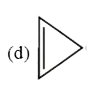
⊕ and CH2=CH−CH+2
View SolutionWhich of the followig pairs has higher resonance energy:
(a).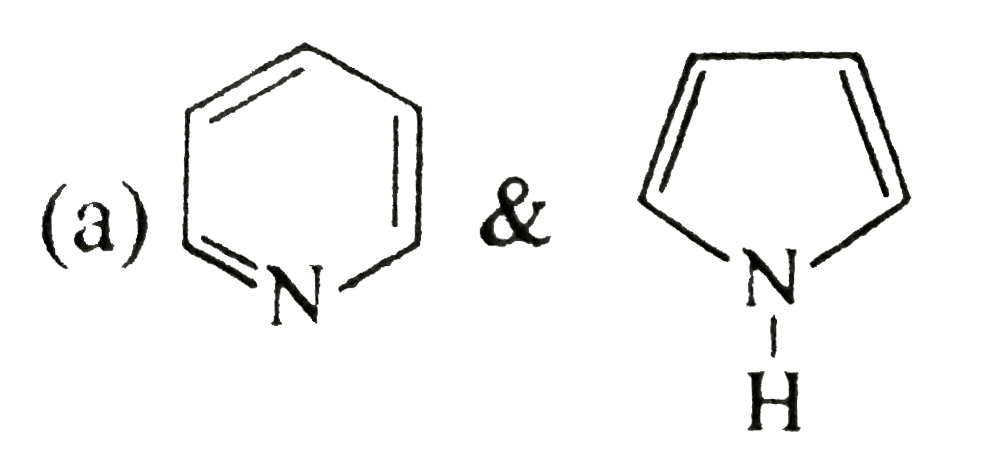
(b). CH2=CH−O−CH=CH2&CH2=CH−NH−CH=CH2
(c). CH2=CH−ΘNH&NH=CH−ΘNH
View SolutionWhich of the following pairs has higher resonance energy :
CH2=CH−OH and CH2=CH−CH=CH−OH
View SolutionCH3CH(OH)CH2CH2COOH
View SolutionWhich of the following is the formula of butanoic acid:
(a) CH3−CH2−CH2−CH2−COOH
(b) CH3−CH2−CH2−CH2−OH
(c) CH3−CH2−CH2−CH2−CH2−COOH
(D) CH3−CH2−CH2−COOH
View SolutionWhich of the following is the correct ease of dehydration ?
I CH3−CH2−CH2−CH2∣OH , II CH3−CH2−OH∣CH−CH3 , III CH2=CH−OH∣CH−CH3, IV
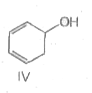
View SolutionCH3−COOH+CH3−OH→CH3−COO−CH3+H2O
View Solution
