Select the incorrect statement.
The geometry of phosphorus in H3PO2,H3PO3 and H3PO34 is tetrahedral
H3PO2,H3PO3 and H3PO4 are tribasic acids
NH3<PH3>AsH3<SbH3: increasing acidic character.
CO2<SiO2<SnO2<PbO2: increasing oxidising power.
The correct Answer is:B
H3PO2,H3PO3 and H3PO4 are having basicity respectively 1, 2 & 3.
Topper's Solved these Questions
Similar Questions
Identify the structure of hydrocarbon (Z) in the given reaction.
C10H16(Z)O3−−→H2O2CH3−COOH+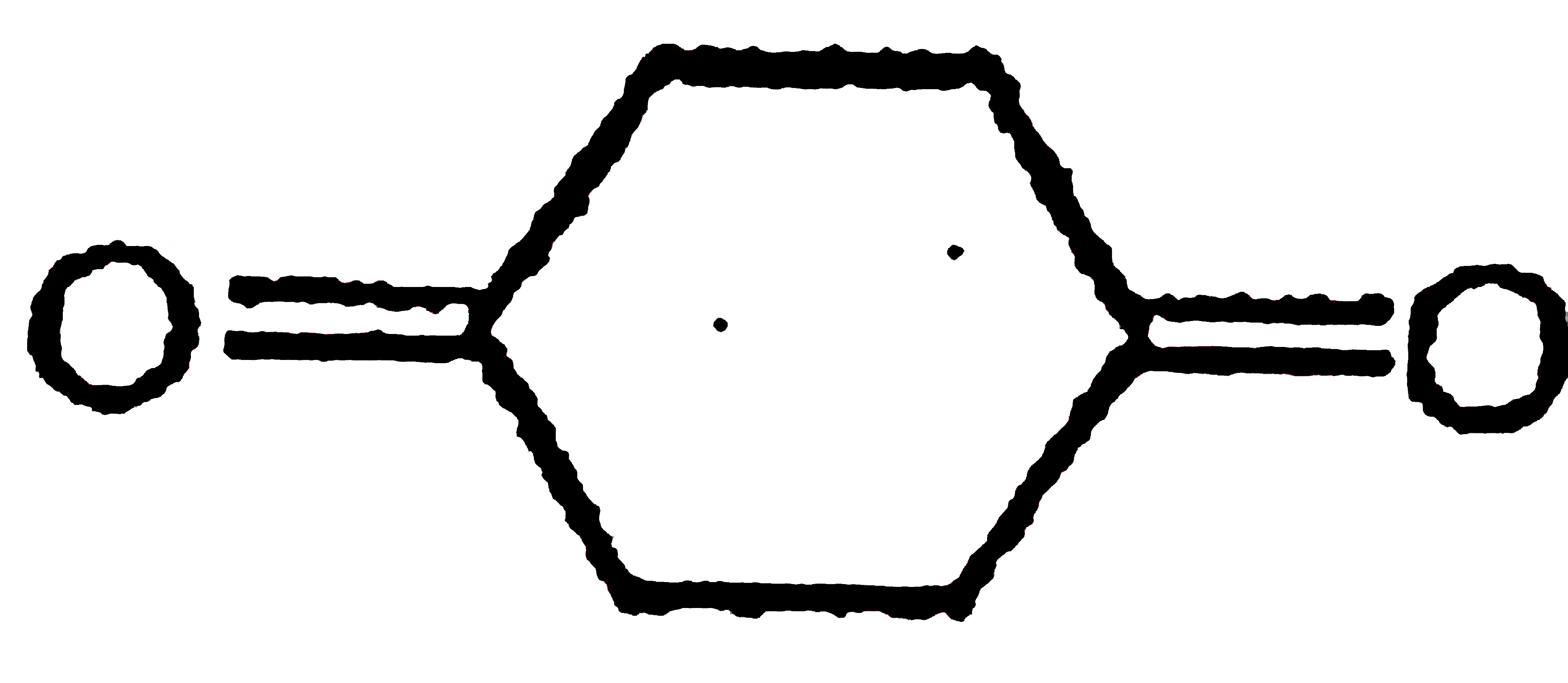
View SolutionGreenhouse gas CO2 can be converted to CO(g) by the following reaction
CO2(g)+H2(g)→CO2+H2O(g) , termed as water gas reaction.
Calculate ΔG for the reaction at 1000K (ΔH1000K=35040Jmol−1ΔS1000K=32.11Jmol−1K1).
View Solution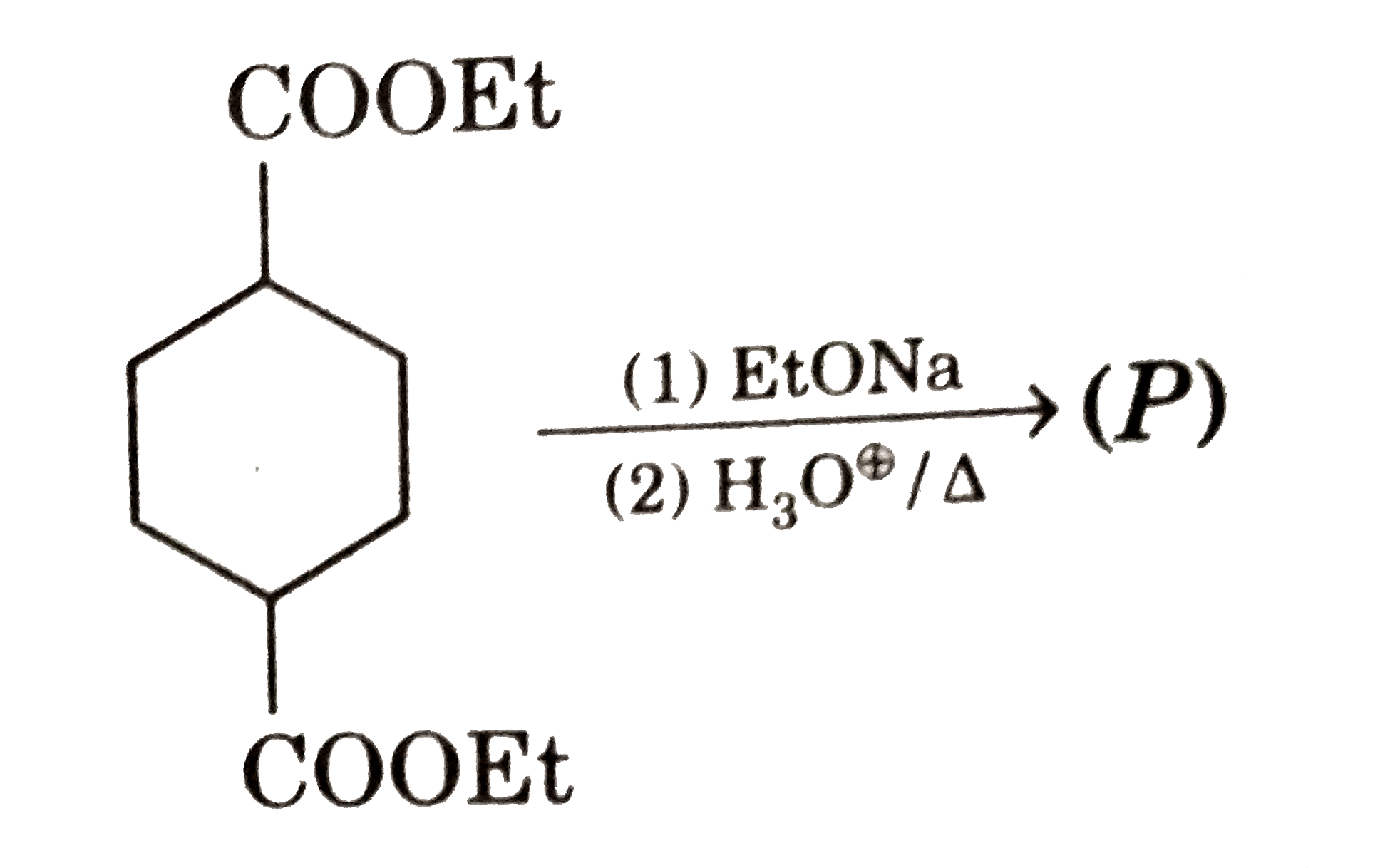
Select incorrect statement :
View SolutionSelect incorrect statements
View SolutionSelect incorrect statement :-
View SolutionSelect incorrect statements
View SolutionSelect incorrect statement:
View SolutionSelect incorrect statement :-
View SolutionSelect the incorrect statement of the following statements.
View SolutionSelect the incorrect statement of the following statements.
View SolutionSelect incorrect statement
View SolutionSelect incorrect statements(s)
View SolutionSelect incorrect statement :
View Solutionअसत्य कथन का चयन कीजिए :-
View SolutionSelect incorrect statements
View SolutionSelect incorrect statement:
View Solution
