Two reaction, (I)A→ Products and (II)B→ Products, follow first order kinetics. The rate of reaction (I) is doubled when the temperature is raised form 300K to 310K. The half life for this reaction at 310K is 30min. At the same temperature B decomposes twice as fast as A. If the energy of activation for reaction (II) is twice that of reaction (I), (a) calculate the rate of constant of reaction (II) at 300K.
The correct Answer is:3.26×10−2−1min
Step by step video, text & image solution for Two reaction, (I)A rarr Products and (II) B rarr Products, follow first order kinetics. The rate of reaction (I) is doubled when the temperature is raised form 300 K to 310K. The half life for this reaction at 310K is 30 min. At the same temperature B decomposes twice as fast as A. If the energy of activation for reaction (II) is twice that of reaction (I), (a) calculate the rate of constant of reaction (II) at 300 K. by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
Similar Questions
Match the reactions in Column I with nature of the reactions/type of the products in Column II
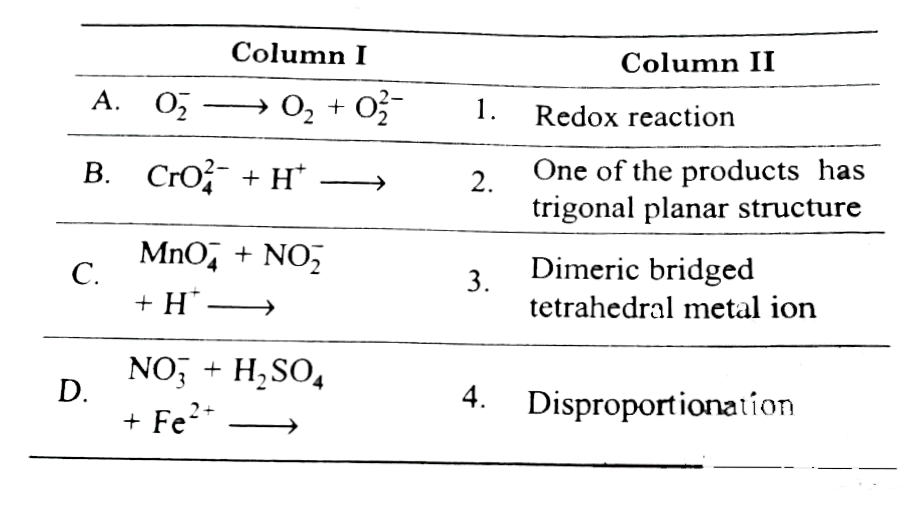
View Solutionदो अभिक्रियाएँ (i) A→ उत्पाद (ii)B→ उत्पाद, प्रथम कोटि के अनुसार होती हैं। जब तापमान 300 K से 310 K तक बढ़ाया जाता है तो अभिक्रिया (i) का वेग दोगुना हो जाता है। इस अभिक्रिया की अर्द्ध-आयु 310K पर 30 मिनट है। इसी ताप पर B का विघटन A की तुलना में दोगुना होता है। यदि अभिक्रिया (ii) की सक्रियण ऊर्जा अभिक्रिया (i) से आधी है, तो अभिक्रिया (ii) का 300 K पर वेग नियतांक क्या होगा?
View Solutionनिम्न दो अभिक्रियाएँ प्रथम कोटि की हैं
(i) A→ उत्पाद (ii) B→ उत्पाद
जब तापमान 300 K से बढ़ाकर 310 K किया जाता है, तो अभिक्रिया (i) का वेग दोगुना हो जाता है। इस अभिक्रिया के लिए, 310K पर अर्द्ध-आयु 30 मिनट है। समान तापक्रम पर, की तुलना में B का वियोजन दोगुना होता है। यदि अभिक्रिया (ii) की सक्रियण ऊर्जा अभिक्रिया (i) की तुलना में आधी हो, तो 300 K पर अभिक्रिया (ii) के लिए वेग स्थिरांक की गणना कीजिए।
View SolutionTwo reactions, (i) A to products and (ii) B to products , follow first order kinetics. The rate of the reaction (i) is doubled when the temperature is raised from 300K to 310K . The half life for this reaction at 310K is 30 minutes. At the same temperature B decomposes twice as fast as A. If the energy of activation for the reaction (i) is half that of reaction (ii) calculate the rate constant of the reaction (ii) at 300K .
View SolutionFor a first order reaction temperature coefficient is 2. If the value of K at 310K is 2×10−2min''−1,t1/2 of reaction at 300K will be (in min)
View SolutionThe rate of a reaction A doubles on increasing the temperature from 300 to 310 K. By how much, the temperature of reaction B should be increased from 300 K so that rate doubles if activation energy of the reaction B is twice to that of reaction A
View SolutionTwo reactions (i)A→P(ii)B→P follow first-order kinetics. The rate of the reaction (i) is doubled when the temperature is raised from 300 K to 310 K. The half-life for this reaction at 310 K is 30 minutes. At the same temperature B decomposes twice as fast as A. If the energy of activation for the reaction (ii) is half that of reaction (i), calculate the rate constant of the reaction (ii) at 300 K.
View SolutionWhat is the energy of activation of a reaction is its rate doubles when the temperature is raised from 290 K to 300 K ?
View Solution

