The difference in the oxidation numbers of the two types of sulphur atoms in Na2S4O6 is
Text Solution
The correct Answer is:5.00
|
Similar Questions
Knowledge Check
The difference of the different oxidation number of the two of sulphur atoms in Na2S2O6 is
A+5B0C+10D−10The two sulphur atoms in Na2S2O3 have
A−2 and +4 oxidation statesB+4 and +6 oxidation statesC+6 and −2 oxidation statesDsame oxidation stateThe average oxidation state of sulphur in Na2S4O6 is
A+6B+32C+52D−2
The difference in the oxidation numbers of two types of sulphul atoms in Na2S4O6 is…..
View SolutionThe difference in the oxidation numbers of two types of sulphul atoms in Na2S4O6 is…..
View SolutionThe difference in the oxidation states of the two types of sulphur atoms in Na2S4O6 is :
View SolutionThe difference in the oxidation numbers of the two types of sulphur atoms in Na2S4O6 is:
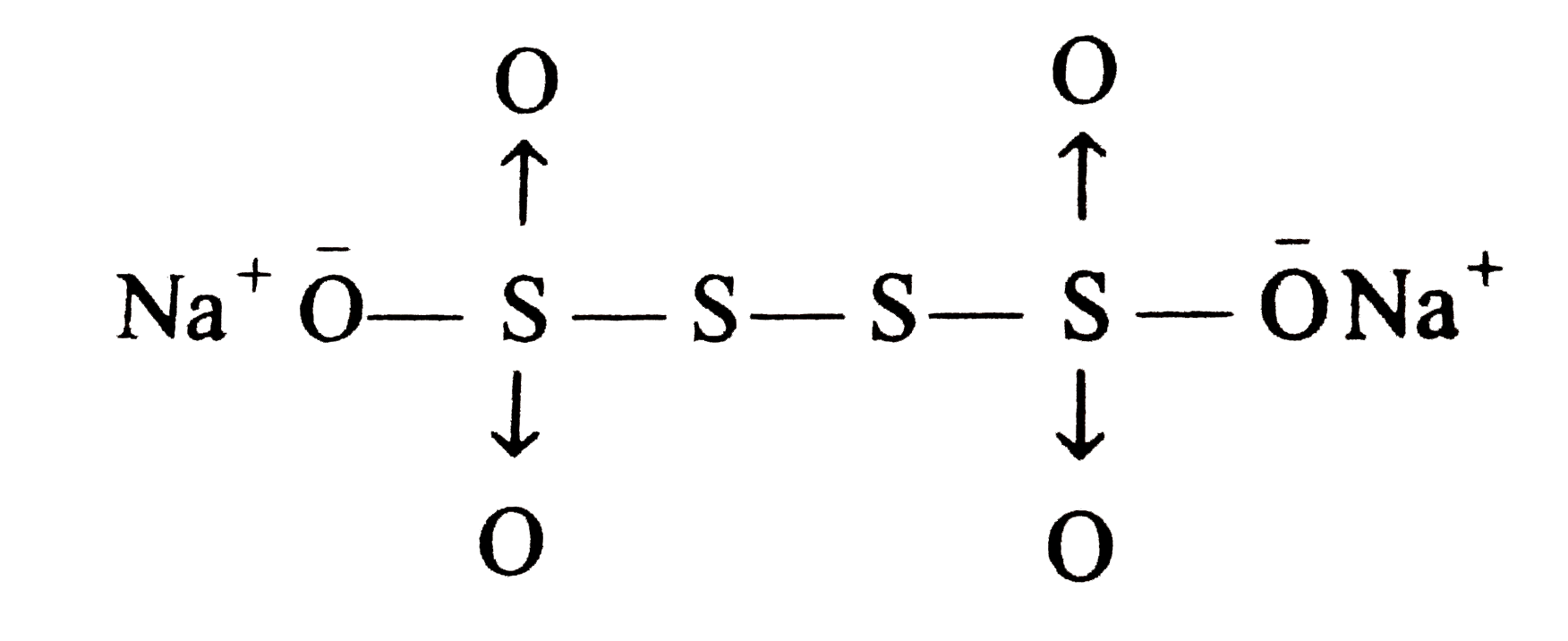
View SolutionThe difference in the oxidation numbers of two types of sulphul atoms in Na2S4O6 is…..
View Solution


