Work function of a metal is 4.0 eV. Find the maximum value of wavelength of a radiation that can emit photoelectrons from the metal.Solution in Bengali
Let, threshold frequency = f0, threshold wavelength = λ0 and work function =W0
∴W0=hf0=hcλ0or,λ0=hcW0
Give, W0=4.0eV=4×1.6×10−12 erg,
we know, h=6.60×10−27 erg.s
and c=3×1010cm.s−1
∴λ0=6.60×10−27×3×10101.6×10−12×4cm
=3.09375×10−5cm≈3094Å
Similar Questions
Radiation of wavelength 310 nm is incidenton a metal surface of work function 1eV . Find maximum speed of photoelectrons emitted . ( Closed value )
View SolutionThe work functions for metals A,B and Care 1.92 eV, 2.0 eV and 5.0 eV respctively. The metals which will emit photoelectrons for a radiation of wavelength 4100Å is / are
View SolutionPhotoelectric work function of a metal is 3 eV. Find the maximum speed of photoelectrons emitted when radiation of wavelength4000∘A is incident on the metal surface. (Given: mass of electron = 9.1×10−31kg)
View SolutionLight of wavelength 3000Å is incident on a metal surface whose work function is 1 eV. The maximum velocity of emitted photoelectron will be
View Solution2eV વર્ક ફંક્શન ધરાવતી ધાતુ પર 4000
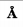 તરંગલંબાઈવાળું વિકિરણ આપાત કરવામાં આવે છે, તો ઉત્સર્જિંત થતા ફોટોઇલેક્ટ્રોનની મહત્તમ ગતિઊ …………………… eV.
તરંગલંબાઈવાળું વિકિરણ આપાત કરવામાં આવે છે, તો ઉત્સર્જિંત થતા ફોટોઇલેક્ટ્રોનની મહત્તમ ગતિઊ …………………… eV.
View SolutionRadiation of wavelength 310 nm is incidenton a metal surface of work function 1eV. Find maximum speed of photoelectrons emitted . ( Closed value )
View SolutionRadiation of wavelength 310 nm is incidenton a metal surface of work function 1eV . Find maximum speed of photoelectrons emitted . ( Closed value )
View SolutionThe maximum kinetic energy of photoelectrons emitted from a metal surface increses from 0.4 eV to 1.2 eV when the frequency of the incident radiation is increased by 40% . What is the work function (in eV) of the metal surface?
View Solution

