If light of wavelength 412.5nm is incident on each of the metals in the table, Which ones will show photoelectric emission and why?
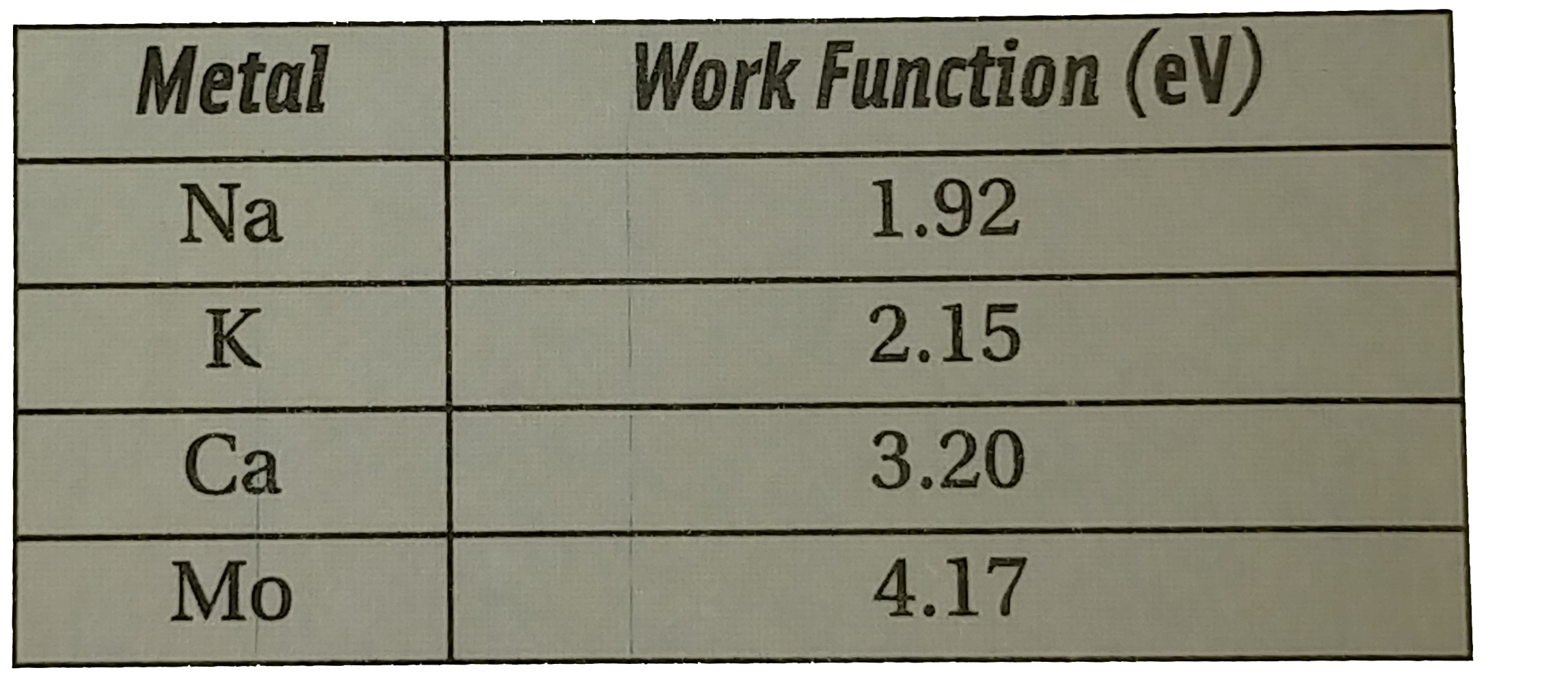
Energy of incident light,
E=12400λ(inÅ)eV=124004125=3eV
Na and K will show photoelectric emission because their work functions are less than the energy of incident light.
Topper's Solved these Questions
- QUANTUM THEORYBOOK - CHHAYA PUBLICATIONCHAPTER - QUANTUM THEORYEXERCISE - Examination Archive with solutions
39 Videos
- OPTICAL INSTRUMENTSBOOK - CHHAYA PUBLICATIONCHAPTER - OPTICAL INSTRUMENTSEXERCISE - CBSE SCANNER
8 Videos
- QUESTION PAPER OF NEET 2017BOOK - CHHAYA PUBLICATIONCHAPTER - QUESTION PAPER OF NEET 2017EXERCISE - QUESTION PAPERS OF (NEET) (2017)
39 Videos
Similar Questions
What will be the kinetic energy of emitted photoelectrons if light of threshold frequency falls on a metal?
View SolutionDefine the magnifying power of a compound microscope when the final image is formed at infinity . Why must both the objective and the eyepiece of a compound microscope has short focal lengths ? Explain.
View SolutionA molecule of a substance has permanent dipole moment p. A mole of this substance is polarised by applying a strong electrostatic field E. The direction of the field is suddenly changed by an angle of 60∘. If N is the Avogadro's number the amount of work done by the field is
View SolutionFor photoelectric emission , tungsten requires light of 2300Å. If light of 1800Å wavelength is incident then emission
View SolutionIf light of wavelength 412.5 nm is incident on each of the metals given below, which one will show photoelectric emission and why? .
View SolutionThe work function for a certain metal is 4.2 eV. Will this metal give photoelectric emission for incident radiation of wavelength 330 nm ?
View SolutionThreshold wavelength for photoelectric emission from a metal surface is 3800Å, Utraviolet light of wave-length 2600Å is incident on the metal surface.
Find the work function of the metal and
View SolutionPhotoelectric threshold wavelength for a metal is 2100Å. If the wavelength of incident radiation be 1800Å, will there be any emission of photoelectrons?
View SolutionThe threshold frequency and the threshold wavelength of photoelectric emission from a metal surface are f_(0) and lamda_(0) . The frequency and the wavelength of incident light are f and lamda . Then
View SolutionIf light of wavelength 412-5 nm is indicent on each of the metals given below , which ones will show photoelectric emission and why?
View SolutionIn an experiment of photoelectric emission for incident light of 4000Å, the stopping potential is 2V. If the wavelength of incident light is made 3000Å, then stopping potential will be :
View SolutionThe work function for a certain metal is 4.2 eV. Will this metal give photoelectric emission for incident radiation of wavelength 330 nm?
View SolutionIn the case of a metal, the starting wavelength of photoelectric action is 2100Å. If the wavelength of incident radiation is 18007, will there be emission of light?
View SolutionThe work function for a certain metal is 4.2 eV. Will this metal give photoelectric emission for incident radiation of wavelength 330 nm?
View SolutionIn photoelectric effect,for emission of electron metal surface ,incident light should have…..
View SolutionThreshold wavelength for lithium metal is 6250 Å wavelength of incident radiation required for photoelectric emission will be……
View Solution

