The spectrum of sodium atom resembles the spectrum of hydrogen atom. In which way is this statement correct ? Solution in Bengali
Video Solution
Text Solution
The correct Answer is:D
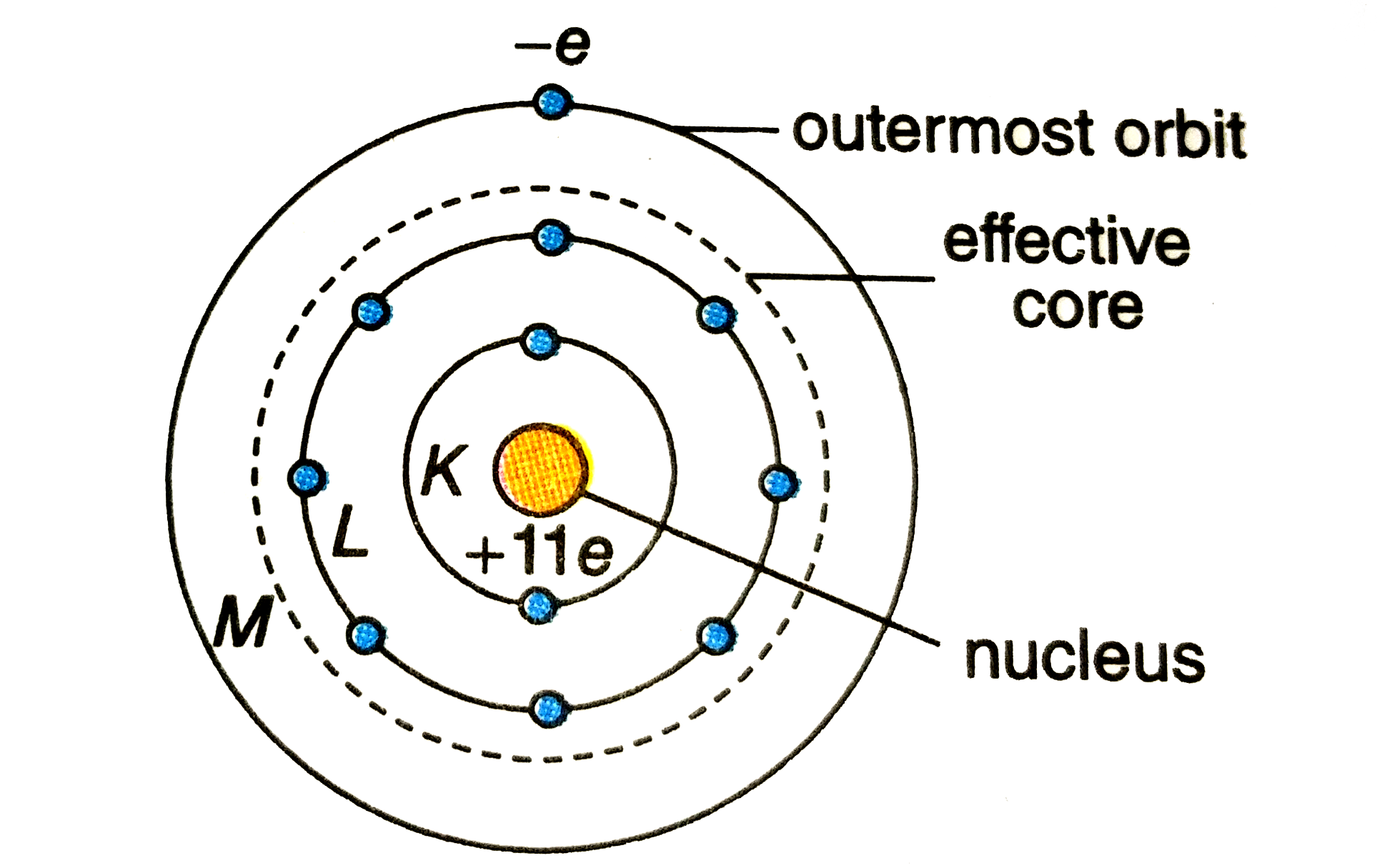
The charge of sodium (Z=11) nucleus = + 11e.
There are 2 and 8 electrons , respectively in its K and L shells [Fig. 1.16] , and there is only one electron in the outermost M -shell .
The nucleus, the K - orbit and the L - orbit in this atom, all together form an effective core and the electron in the M - orbit revolves round this core. Moreover, the charge of the core = 11e (2+8) e =+e charge of H-nucleus.
So, this electron in the M- orbit resembles the electron revolving round the nucleus in a hydrogen atom. thus , the spectrum of sodium atom resembles the spectrum of hydrogen atom.
|
Similar Questions
In hydrogen atom spectrum
View SolutionName the five series in the atomic spectrum of hydrogen.
View SolutionIn what sense is the spectrum of the sodium atom similar to the spectrum of the hydrogen atom?
View Solutionহাইড্রোজেন পরমাণু বর্ণালিতে
View SolutionAtomic Spectra|Emission Spectrum of Hydrogen
View Solutionहाइड्रोजन परमाणु के स्पेक्ट्रम के लिए बोर मॉडल
View SolutionWhich statement is /are correct about hydrogen spectrum?
View SolutionWhich of the following statements about hydrogen spectrum are correct?
View Solution

