For two Bohr orbits n1andn2 which follow the relationships, (n22=n21)=21and(n2−n1)=3.
If an electron makes transition from n2ton1 directly then :
third longest wavelength of Balmer series is emitted
longest frequency of Lyman series is amitted
longest frequency of Balmer saries is emitted
only one spectral line is emitted
The correct Answer is:a d
Step by step video, text & image solution for For two Bohr orbits n_(1) "and"n_(2) which follow the relationships, (n_(2)^(2)=n_(1)^(2))=21 and (n_(2) - n_(1))=3. If an electron makes transition from n_(2) "to" n_(1) directly then : by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Similar Questions
Knowledge Check
There is transition from n=1 to n=2 and then n=2 to n=3, then
AδE value as well as frequency are additiveBwavelenght as well as frequency are additiveCΔE values as well as wavelength are additiveDall the parameters, (ΔE, frequency and wavelength are additiveThe electronic transition from n = 2 to n = 1 will produce shortest wave length in (where n = principle quantum state)
ALi2+BHe+CHDH+For the Paschen series the values of n1 and n2 in the expression ΔE=RH.c(1n21−1n22)
An1=1,n2=2,3,4 …Bn1=2,n2=3,4,5Cn13,n2=4,5,6 …….Dn1=4,n2=5,6,7 ……
Find the integral solution for n1n2=2n1−n2, where n1,n2∈integer.
View SolutionConsider the follwoing two elerctronic transition possibilites in a hydrogen atom as pictured below :
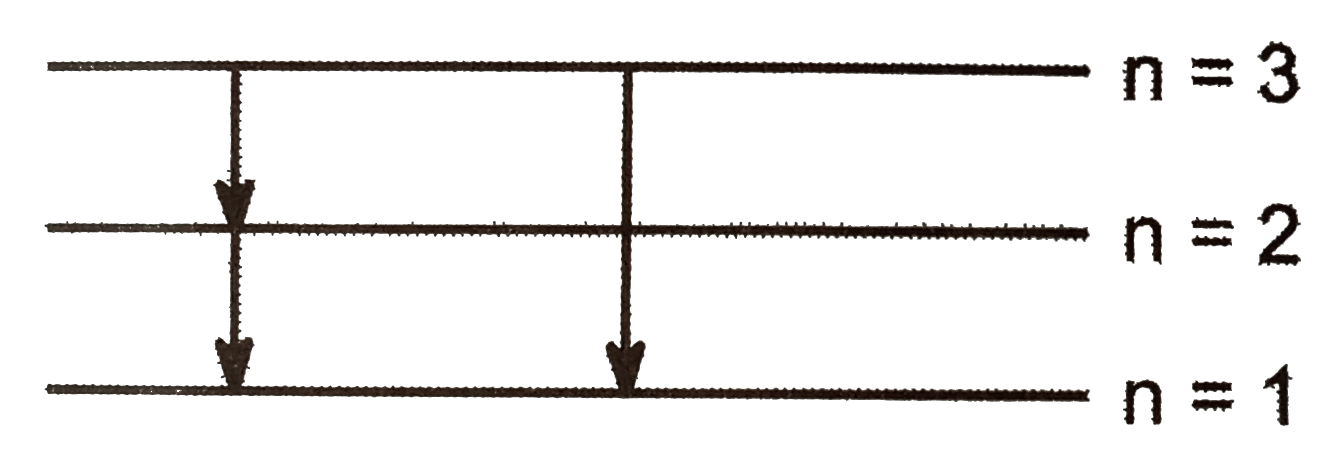
(1) The electron drops from third Bohr's orbit to second Bohr's obit followed with the next transition from second to first Bohr's orbit .
(2) The electron drops from third Bohr's orbit to first Bohr's orbit directly .
Show that :
(a) The sum of the enrgies for the transitions n=3 to n=2 and n=2 to n=1 is equal to the energy of transiton for n=3 to n=1 .
(b) Are wavelengths and frequencies of the emitted spectrum also additive in the same way as their energies are ?
View Solutionn1andn2 are four-digit numbers, find the total number of ways of forming n1andn2 so that n2 can be subtracted from n1 without borrowing at any stage.
View SolutionThe transition of an electron from n2=5,6….. To n1=4 gives rise to
View SolutionWhich one of the following is/are correct relation?
n21=1n12
n32=n31×n12
n21=n1an2a
n21=n2an1a
View Solution

