For a H-like atom which Bohr's model some spectral lines were observed as shown If it is known that line E belongs to visible region then the correct statement(s) is/are for following transition.
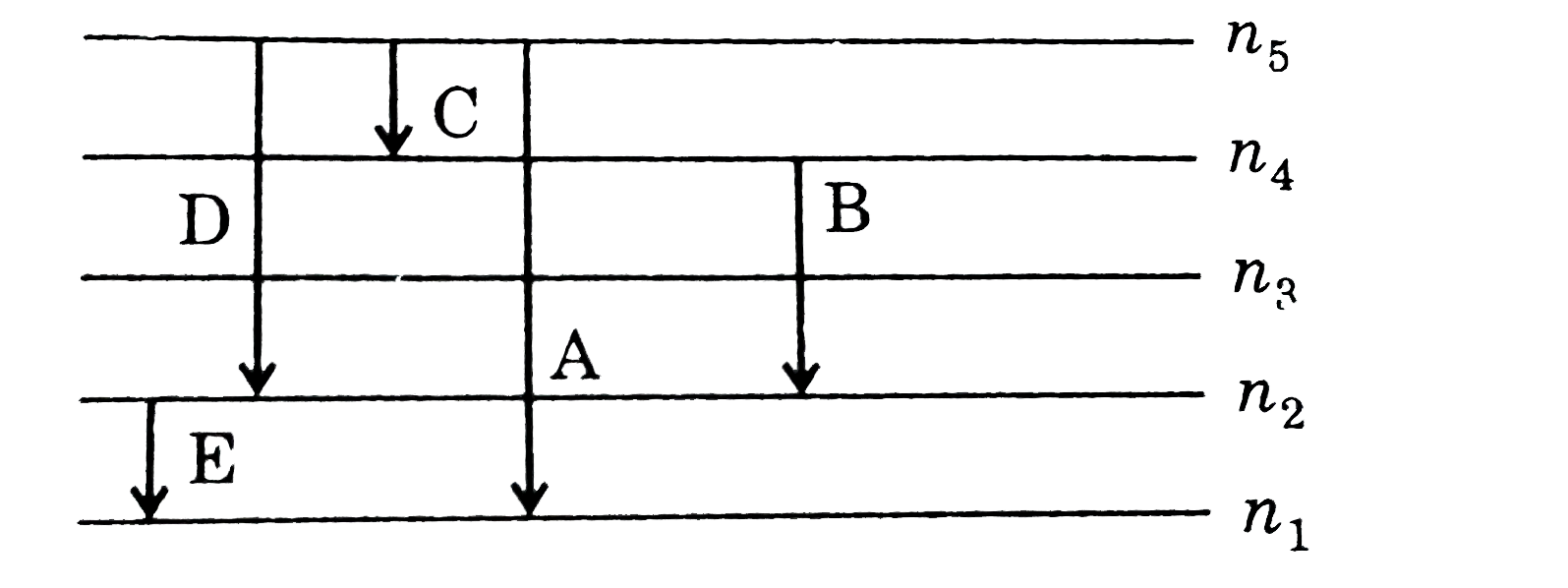
There cannot be any line in UV region
only line possibly belonging to ultraviolet region is A
Line having shortest wavelength is A
Line having least energy is C.
The correct Answer is:c d
Step by step video, text & image solution for For a H-like atom which Bohr's model some spectral lines were observed as shown If it is known that line E belongs to visible region then the correct statement(s) is//are for following transition. by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Similar Questions
The number of lines of Balmer series of H-atom that belong to visible region.
View SolutionHydrogen atoms are in an excited state with n = 5. In terms of the Bohr's model, (a) how many spectral lines can be radiated as these atoms return to be ground state? (b) Find the energies of the line with the longest and shortest wavelengths.
View Solution
Knowledge Check
For a hypothetical H like atom which follows Bohr's model, some spectral lines were observed as shown. If it is known that line 'E' belongs to the visible region, then the lines possibly belonging to ultraviolet region will be (n1 is not necessarily ground state). [Assume for this atom, no spectral series shows overlaps with other series in the emission spectrum
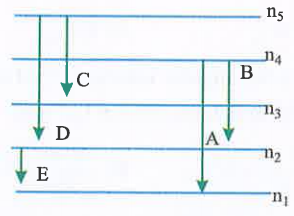 AB and DBD onlyCC onlyDA only
AB and DBD onlyCC onlyDA onlyFor a hypothetical H like atom which follows Bohr's model, some spectral lines were observed as shown.If it is known that line 'E' belongs to the visible region, then the lines possibly belonging to ultra violet region will be (n1 is not necessarily ground state )
[Assume for this atom, no spectral series shows overlap with other series in the emmission spectrum]
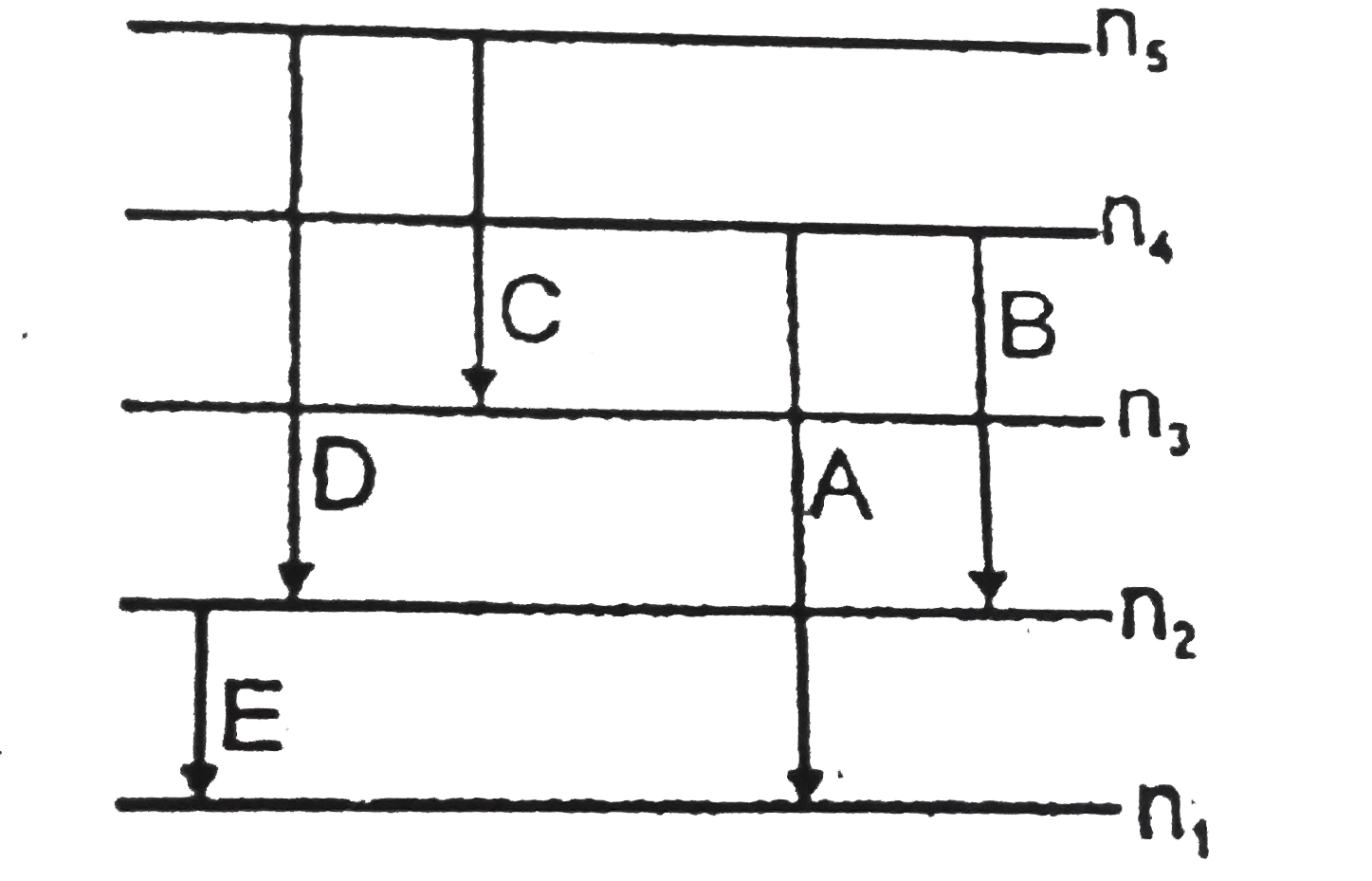 AB and DBD onlyCC onlyDA only
AB and DBD onlyCC onlyDA onlyWhich spectral lines are in visible region?
ALyman seriesBBalmer seriesCPaschen seriesDNone of these
Total different spectral lines observed in between 11th excited state and 3rd energy level in H-atom emission spectrum are:
View SolutionWhich of the following series of H-atom lies in visible range?
View SolutionIdentify the correct statement(s).
The findings from the Bohr model for H-atom are
View SolutionHow many spectral lines are seen for hydrogen atom when electron jump from n2=5 to n1=1 in visible region ?
View SolutionWhich of the following spectral series in hydrogen atom gives spectral line of wavelenght 486 nm ?
View Solution

