Two glass bulbs A (of 100 mL capacity), and B (of 150mL capacity) containing same gas are connected by a small tube of negligible volume. At particular temperature the pressure to A was found to be 20 times more than that in bulb B. the stopcock is opened without changing the temperature. the pressure in A will:
drop by 75%
drop by 57%
drop by 25%
will remain same
The correct Answer is:b
Similar Questions
Two cylinders A and B of equal capacity are connected to each other vis a stop cock. A contains an ideal gas at standard temperature and pressure. B is completely evacuated. The sto cock is suddenly opened. The process is:
View SolutionInitially bulb "a" contained oxygen gas at 27∘C and 950 mm of Hg and bulb "B" contained neon gas at 27∘C and 900 mm of Hg. These bulbs are connected by a narrow tube of negligible volume equipped with a stopcock and gases were allowed to mix-up freely. The pressure obtained in the combined system was found to be 910 mm of Hg.
If volume of bulb B was measured to be 10 L, then find the volume of oxygen gas present initially in bulb "A" .
View Solution
Knowledge Check
Two vessels A and B contain the same gas. If the pressure, volume and absolute temperature of the gas in A are two times as compared to that in B, and if the mass of the gas in B is x grams, the mass of the gas in A will be
A4x gBx2 gC2x gDx gIf two gases are taken at same temperature, the density of a gas A is three times that of gas B, while molecular mass of gas B is twice that of A. The ratio of pressure of A and B will be:
A16B6C23D32Two flasks A and B of equal capacity of volume contain SO3 and CO gas respectively under similar conditions of temperature and pressure. Then, which of the following statement is true?
AA has twice the number of moles as that of BBB has twice the number of moles as that of ACA and B have equal number of molesDB has twice the number of atoms as that of A
A Vessel of 120mL capacity contains a certain mass of gas at 20∘C and 75mm pressure. The gas was transferred to a vessel whose volume is 180mL. Calculate the pressure of the gas at 20∘C
View SolutionA Vessel of 120mL capacity contains a certain mass of gas at 20∘C and 75mm pressure. The gas was transferred to a vessel whose volume is 180mL. Calculate the pressure of the gas at 202C
View Solution200 mL of a gas are found to have a pressure of 750 mm. What will be its volume if the pressure is doubled at the same temperature ?
View SolutionA thin glass bulb of 100 mL capacity is evacuated and kept in 2.0 L container at 27∘C and 800 mm pressure. If the bulb implodes isothermally, calculate the new pressure in the container in kilopascals (kPa)
View SolutionTwo gas bulbs are connected by a thin tube. Calaculate the partial pressure of helium after the connective value is opened at a constant temperature of 27∘C
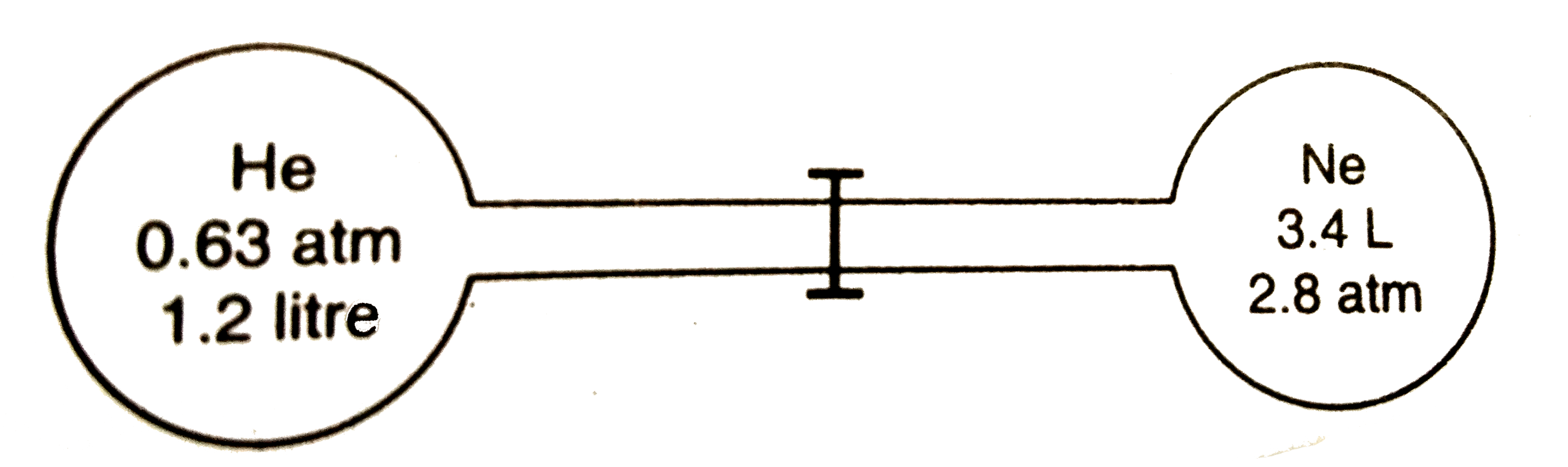
View Solution

