Water and turpentine oil (of specific heat less than that of water) are both heated to same temperature. Equal amounts of these placed in indentical claorimeters are then left in air.
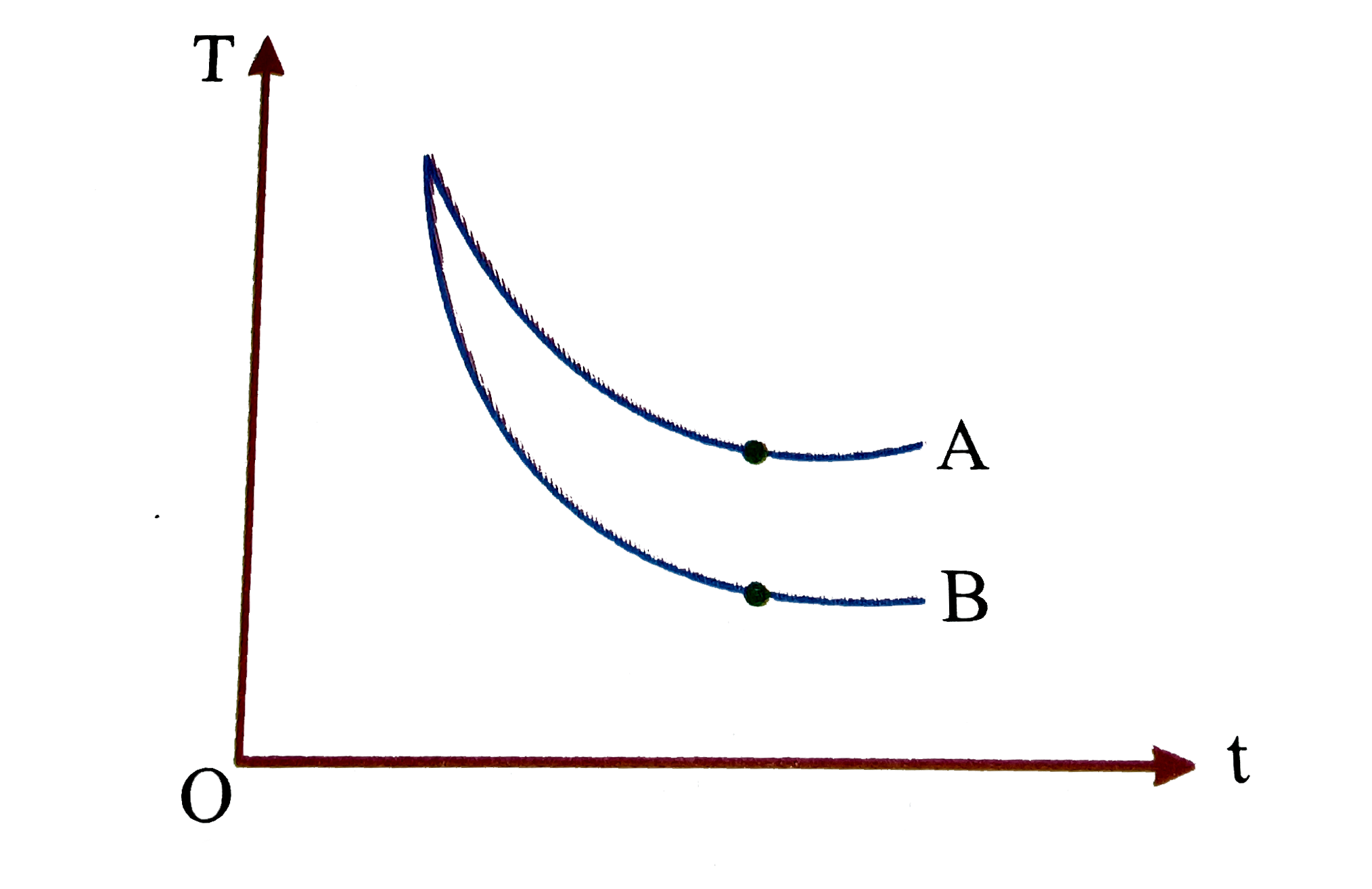
Their cooling curves will be identical
A and B will represent cooling curves of water and oil respectively
B and A will represent cooling curves of water and oil respectively
None of the above
The correct Answer is:B
Step by step video, text & image solution for Water and turpentine oil (of specific heat less than that of water) are both heated to same temperature. Equal amounts of these placed in indentical claorimeters are then left in air. by Physics experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
- TRANSMISSION OF HEATBOOK - ERRORLESS CHAPTER - TRANSMISSION OF HEATEXERCISE - Assertion & Reason
24 Videos
- THERMOMETRY, THERMAL EXPANSION AND CALORIMETRY BOOK - ERRORLESS CHAPTER - THERMOMETRY, THERMAL EXPANSION AND CALORIMETRY EXERCISE - Self Evaluation Test
15 Videos
- UNITS, DIMENSION & MEASUREMENTSBOOK - ERRORLESS CHAPTER - UNITS, DIMENSION & MEASUREMENTSEXERCISE - All Questions
333 Videos
Similar Questions
Assertion : A body that is a good radiator is also a good absorber of radiation at a given wavelength.
Reason : According to Kirchhoff's law the absorptivity of a body is equal to its emissivity at a given wavelength
View SolutionOut of the following, in which vessel will the temperature of the solution be higher after the salt is completely dissolved.

View SolutionLight year is a unit of
View SolutionWater and turpentine oil (of specific heat less than that of water) are both heated to same temperature. Equal amounts of these placed in indentical claorimeters are then left in air.

View SolutionA 10 W electric heater is used to heat a container filled with 0.5 kg of water. It is found that the temperature of water and container rises by 3^(@) K in 15 min. The container is then emptied, dired and filled with 2kg of oil. The same heater now raises the temperature of container oil system by 2K in 20 min. Assume there is no heat loss in the process and the specific heat of water is 4200J kgi^(-1)K%^(-1) , the specific heat of oil in the same limit is equal to
View SolutionCalculate the amount of heat required to convert 80g of ice at 0∘C into water at the same temperature . ( Specific latent heat of fusion of ice =80cal/g )
View SolutionA 10 Watt electric heater is used to heat a container filled with 0.5 kg of water. It is found that the temperature of water and the container rises by 3, K in 15 minutes. The container is then emptied, dried and filled with 2 kg of oil. The same heater now raises the temperature of container-oil system by 2''K in 20 minutes. Assuming that there is no heat loss in the process and the specific heat of water as "4200 J . kg"^(-1).K^(-1) , the specific heat of oil in the same unit is equal to
View Solutionకిరోసిన్, టర్పంటైన్ ఆయిల్ మరియు నీరులలో కాంతి వేగం దేనిలో ఎక్కువ ?
View SolutionWater and turpentine oil (both specific heat less than water are heated to the same temperature. Now the same amount of both is taken separately in two identical calorimeters and placed in the air, then
View SolutionA 10 watt electric heater is used to heat a container filled with 0.5 kg of water. It is found that the temperature of water and the container rises by 3∘K in 15 minutes. The container is then emptied, dried and filled with 2 kg of oil. The same heater now raises the temperature of container-oil system by 2∘K in 20 minutes. Assuming that there is no heat loss in the process and the specific heat of water as 4200Jkg−1K−1 the specific heat of oil in the same unit is equal to
View SolutionAt which temperature specific heat of water is minimum ?
View SolutionA 10 Watt electric heater is used to heat a container filled with 0.5 kg of water. It is found that the temperature of water and the container rises by 3^@ K in 15 minutes. The container is then emptied, dried and filled with 2 kg of oil. The same heater now raises the temperature of container-oil system by 2^@ K in 20 minutes. Assuming that there is no heat loss in the process and the specific heat of water as 4200 Jkg^(-1) k^(-1) , the specific heat of oil in the same unit is equal to
View SolutionA 10 W electric heater is used to heat a container filled with 0.5 kg of water. It is found that the temperature of water and container rises by 3∘K in 15 min. The container is then emptied, dired and filled with 2kg of oil. The same heater now raises the temperature of container oil system by 2K in 20 min. Assume there is no heat loss in the process and the specific heat of water is 4200Jkg−1K−1, the specific heat of oil in the same limit is equal to
View SolutionA 10 Watt electric heater is used to heat a container filled with 0.5 kg of water. It is found that the temperature of water and the container rises by 3 K in 15 minutes. The container is then emptied, dried and filled with 2 kg of oil. The same heater now raises the temperature of container-oil system by 2 K in 20 minutes. Assuming that there is no heat loss in the process and the specific heat of water as 4200Jkg−1K−1,the specific heat of oil in the same unit is equal to
View SolutionSome heat energy is given to 120 g of water and its temperature rises by 10 K. When the same amount of heat energy is given to 60 g of oil, its temperature rises by 40 K. The specific heat capacity of water is 4200 J kg- K^(-1) . Calculate : (i) the amount of heat energy in joule given to water, and (ii) the specific heat capacity of oil.
View Solution50 g of oil "A" and 100 g of oil "B" are taken in separate containers at same temperature and specific heat of oil "B" is double that of oil "A." Both are heated in such a way that increase in temperature is also the same. Calculate the ratio of heat required to be supplied for increasing the temperature.
View Solution

