C6H6+Br2→C6H5Br+HBr
In an experiment, to prepare bromobenzene according to the equation, a student reacted 20.0g of C6H6 with 0.310 mol of bromine. If 28.0 g of C6H5Br was obtained, what was the percentage yield?
31.5
40.3
57.6
69.7
The correct Answer is:D
Step by step video, text & image solution for C_(6)H_(6) + Br_(2) rightarrow C_(6)H_(5)Br + HBr In an experiment, to prepare bromobenzene according to the equation, a student reacted 20.0g of C_(6)H_(6) with 0.310 mol of bromine. If 28.0 g of C_(6)H_(5)Br was obtained, what was the percentage yield? by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Similar Questions
Knowledge Check
C3H6Br2 can shown:
Atwo gem dibromideBtwo vic dibromideCfour total dibromo derivativesDall of the aboveC6H13Br+OH−→C6H13OH+Br− is an example of :
ANucleophilic additionBNucleophilic substitionCElectrophillic addtionDElectrophilic substitionMethyl benzoate is prepared by the reaction between benzoic acid and methanol, according to the equation
C6H5COOH+CH3OH→C6H5COOCH3+H2O
In an experiment 24.4gm of benzoic acid were reacted with 70.0mL of CH3OH. The density of CH3OH is 0.79 g mL−1. The methyl benzoate produced had a mass of 21.6g. What is the percentage yeild of product?A0.917B0.794C0.715D0.217
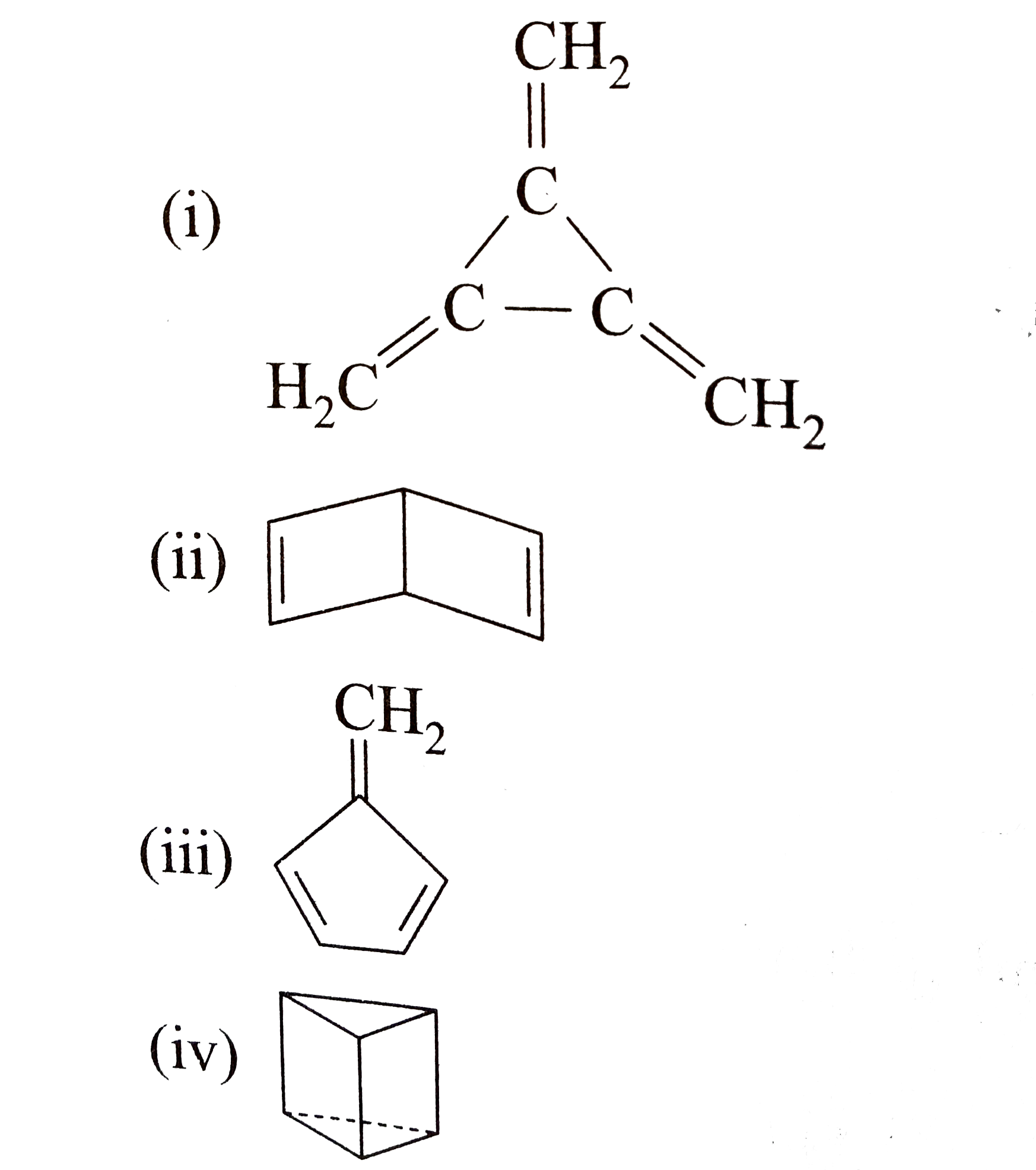
Which of the followng C6H6 structures give only one C6H5Br isomer?
View SolutionWhich of the following C6H6 structures will not give only one C6H5Br isomer
View SolutionThe mole fraction of a given sample of I2 in C6H6 is 0.2. The molality of I2 in C6H6 is:
View SolutionBenzene forms a single C6H5Br product when it is monobrominated because
View SolutionReaction of C6H5CH2Br with aqueous sodium hydroxide follows _____ .
View Solution

