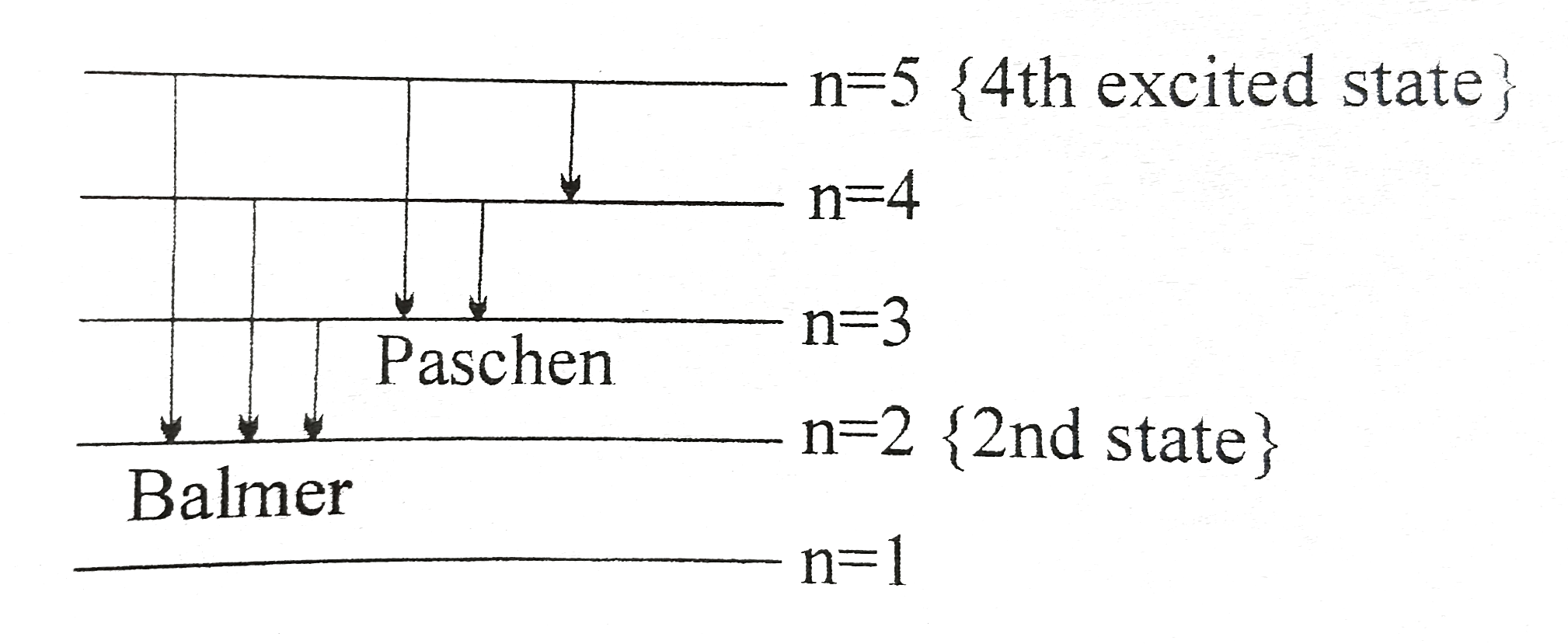In a H- like sample electron makes transition from 4th excited state of 2nd state then:
10 different spectral lines are observed
6 different spectral lines are observed
Number of line belonging to the the Blamer series id 3.
Number of line belonging to the the Blamer series id 2.
The correct Answer is:B, C, D
Similar Questions
For the hydrogen atom, the energy of radiation emitted in the transition from 4th excited state to 2nd excited state, according to Bohr's theory is
View SolutionAs an electron makes a transition from an excited state to the ground state of a hydrogen -like atom / ion
View SolutionAs an electron makes a transition from an excited State to the ground state of a hydrogen - like atom/ion :
View SolutionAs an electron makes a transition from an excited state to the ground state of a hydrogen - like atom /ion
View SolutionAs an electron makes a transition from an excited state to the ground state of a hydrogen-like atom ion. The wrong option(s) is (are)
View SolutionAs an electron makes a transition from an excited state to the ground state of a hydrogen like atom/ion.
View SolutionIn a H like sample electrons make transition from 4th excited state to 1st state then
View SolutionIf electron make transition from 7th excited state to 2nd state in H atom sample find the max. number of spectral lines observed.
View Solution