Match the column -
Column-IColumn-II(A)NaOH+SO2→(p)NaNO3(B)NaOH+CO2→(q)Na2SO3(C)NaOH+NO2→(r)Na2CO3(D)NaOH+HNO3→(s)NaNO2
The correct Answer is:A-q, B-r, C-p, s, D-p
Step by step video, text & image solution for Match the column - {:(,"Column-I",,"Column-II"),((A),NaOH+SO_(2) rarr,(p),NaNO_(3)),((B),NaOH+CO_(2) rarr,(q),Na_(2)SO_(3)),((C),NaOH+NO_(2) rarr,(r),Na_(2)CO_(3)),((D),NaOH+HNO_(3) rarr,(s),NaNO_(2)):} by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
Similar Questions
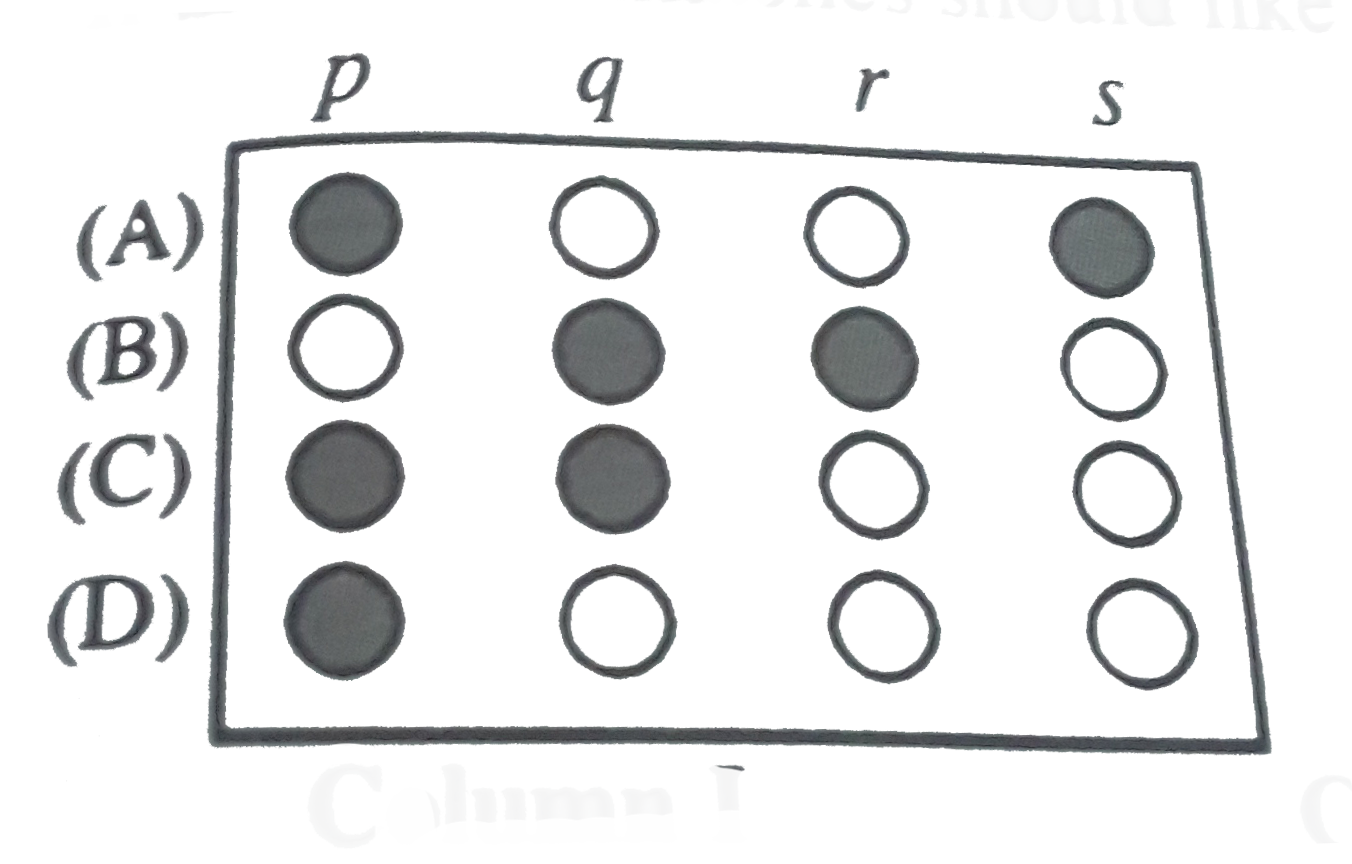
Here each question contains statements given in two column which have to be matched. Statements in columns I are labelled as A, B, C and D where as the statements in column II are labelled p, q, r and s. The answers to these questions are to be appropriately bubbled as illustrated in the following example.
If the correct matches should like the following.
Column IColumn II(A)SO2(p)Basic(B)H2SO4(q)Acidic(C)HNO3(r)Reducing(D)NH3(s)Oxidizing
View Solutionwhich one of the following reaction is not associated with the solvay process of manufacture of Na2CO3? (A) NaCl+NH4HCO3→ NaHCO3+NH4Cl
(B) 2NaOH+CO2 → Na2CO3+H2O
(C) 2NaHCO3→ Na2CO3+H2O+CO2
(D) NH3+H2CO3 → NH4HCO3
View Solution
Knowledge Check
Na2CO3+Pb(NO3)2→PbCO3⏐↓+NaNO3
AFor coloured ppt./Black pptBFor coloured solution.Cfor clear/colourless solutionDFor white ppt.Na2CO3+AgNO3→Ag2CO3↓+NaNO3
AFor coloured ppt./Black pptBFor coloured solution.Cfor clear/colourless solutionDFor white ppt.Na2CO3+AgNO3→Ag2CO3↓+NaNO3
AFor coloured ppt./Black pptBFor coloured solution.Cfor clear/colourless solutionDFor white ppt.
Match the transformation in column I with appropriate option in column II
Column IColumn II(A)CO2(s)→CO2(g)(p)phase transition(B)CaCO3(s)→CaO(s)+CO2(g)(q)allotropic change(C)2H⋅→H2(g)(r)ΔH is positive(D)P(white, solid)→P(red, solid)(s)ΔS is positive(t)ΔS is negative
View SolutionMatch Column -I with Column -II
Column-IColumn-II(A)Sorel's cement(p)MgCl2(B)Albite(q)MgO(C)A salt of carnallite(r)NaAlSl3O8(D)Glauber's salt(s)Na2SO4.10H2O
View SolutionMatch the reacting mixture in column-I with the reagent in column II
Column-IColumn-II(A)H2C2O4+NaHC2O4(p)NaOH(B)NaHPO4+NaNO2(q)HCl(C)Fe2(SO4)3+FeC2O4(r)KMnO4(D)FeO+Fe2O3(s)Zinc dust
View SolutionMatch the column -
Column-IColumn-IIFor the production of H2O2(A)Down process(p)K2CO3(B)Solvay process(q)Manufacture of Na(C)Leblance process(r)Manufacture of NaOH(D)Castner- K ellner cell(s)Na2CO3
View SolutionBa(NO3)2+Na2SO4→BaSO4⏐↓+2NaNO3
View Solution
