A solid with a formula AB has the rock-salt type of structure. If all the atoms touching one body-diagonal plane are removed, then what would the formula of the compound be?Solution in Bengali
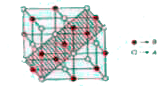
If the unit cell of AB contains A atoms at its corners and face centres, the B atoms will be situated at its edge centres and body centres. A body-diagonal plane of the unit cell is associated with four A atoms at four corners, two A atoms at the centres of the opposite faces, two B atoms at the edge centres of the diagonally opposite edges and one B atom at the body centre.
Removing four A atoms from the four corners and two A atoms from face centres leaves a total of 4×18+4×12=52 A atoms in the unit cell. On the other hand, removal of two B atoms from two edge centres and body centre leaves a total of two 10×14=52 B atoms in the unit cell. Thus the ratio of A to B atoms per unit cell will become
A:B=52:52=1:1
So, the formula of the compound remains as AB.
If the locations of A and B atoms are considered to be the other way around, that is, B atoms are at the corners and face centres, and A atoms at the edge centres and body centre, then the formula of the compound will also remain as AB.
Similar Questions
In a crystalline solid, atom of A form fcc packing and atoms of B occupy all octahedral voids. If all the atoms along one body diagonal are removed then what will be the simplest formula of the crystalline solid ?
View SolutionIn a unit cell, atoms A, B, C and D are present at corners, face - centres, body - centre and edge - centres respectively. If atoms touching one of the plane passing through two diagonally opposite edges are removed, then formula of compound is
View SolutionAn Ionic Solid A^(+)B^(-) Crstalise like rock salt if all atoms along one body diagonal are removed then what is the formula of substance ?
View SolutionIf all the atoms, on the shaded plane are removed then the molecular formula of the solid will be
View SolutionA solid AB forms rock salt structure in which anions B− forms the lattice. If all particles along one of the tetrad axis is removed the formula of resulting compound is
View SolutionIn a solid having rock salt structure, if all tge atoms touching one body diagonal plane are removed (except at body centre), then the formula of the left unit cell is
View SolutionIn a unit cell, atoms A,B,C and D are present at corners, face-centres, body-centre and edge-centres respectively. If atoms touching one of the plane passing through two diagonally opposite edges are removed, then formula of compound is:
View SolutionIn a unit cell, atoms A, B, C and D are present at corners, face - centres, body - centre and edge - centres respectively. If atoms touching one of the plane passing through two diagonally opposite edges are removed, then formula of compound is
View Solution
