The aqueous solution of NH3 is slightly basic whereas aqueous solution of H2S. Is slightly acidic. Why?Solution in Bengali
Step by step video & image solution for The aqueous solution of NH_(3) is slightly basic whereas aqueous solution of H_(2)S. Is slightly acidic. Why? by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Similar Questions
HCl का जलीय विलयन अम्लीय क्यों होता है ?
View SolutionThe order of basic strength of methyl substituted amines and NH3 in aqueous solution is
View SolutionAqueous HCL is an acidic solution whereas aqueous NH_3 is a basic solution. Justify this statement.
View Solution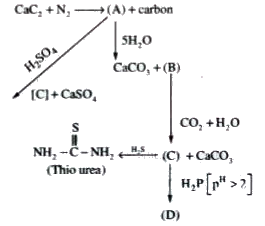
'B' is a weak basic aqueous NH3 solution
View Solutionकथन : NH4NO3 के जलीय विलयन की प्रकर्ति अम्लीय होती है
कारण : NH4NO3 जलीय विलयन में ऋणायनिक जल अपघटन दर्शाता है
View SolutionWhy are aqueous solutions of amines basic in nature ?
View SolutionWhy an aqueous solution of NH_4CI is acidic while that of HCOOK is basic?
View SolutionA solution of NH4F is slightly acidic. Why?
View Solution

