(i) Plotting V vs T for a given mass of an ideal gas at two different fixed pressures P1andP2 gives two straight lines, one of which corresponds to P1 and the other corresponds to P2. If P1 is greater than P2, then which one of thes straight lines will make a larger angle with the T-axis?
(ii) Plotting P vs T for a given mass of an ideal gas at two different fixed volumes V1andV2 gives two straight lines, one of which corresponds to V1 and the other corresponds to V2. if V1 is greater than V2, then which one of these straight lines will make a larger angle with the T-axis?Solution in Bengali
(i) Straight line obtained at pressure P2 will make larger angle with the T-axis.
(ii) The straight line obtained at volume V2 will make larger angle with the T-axis.
Similar Questions
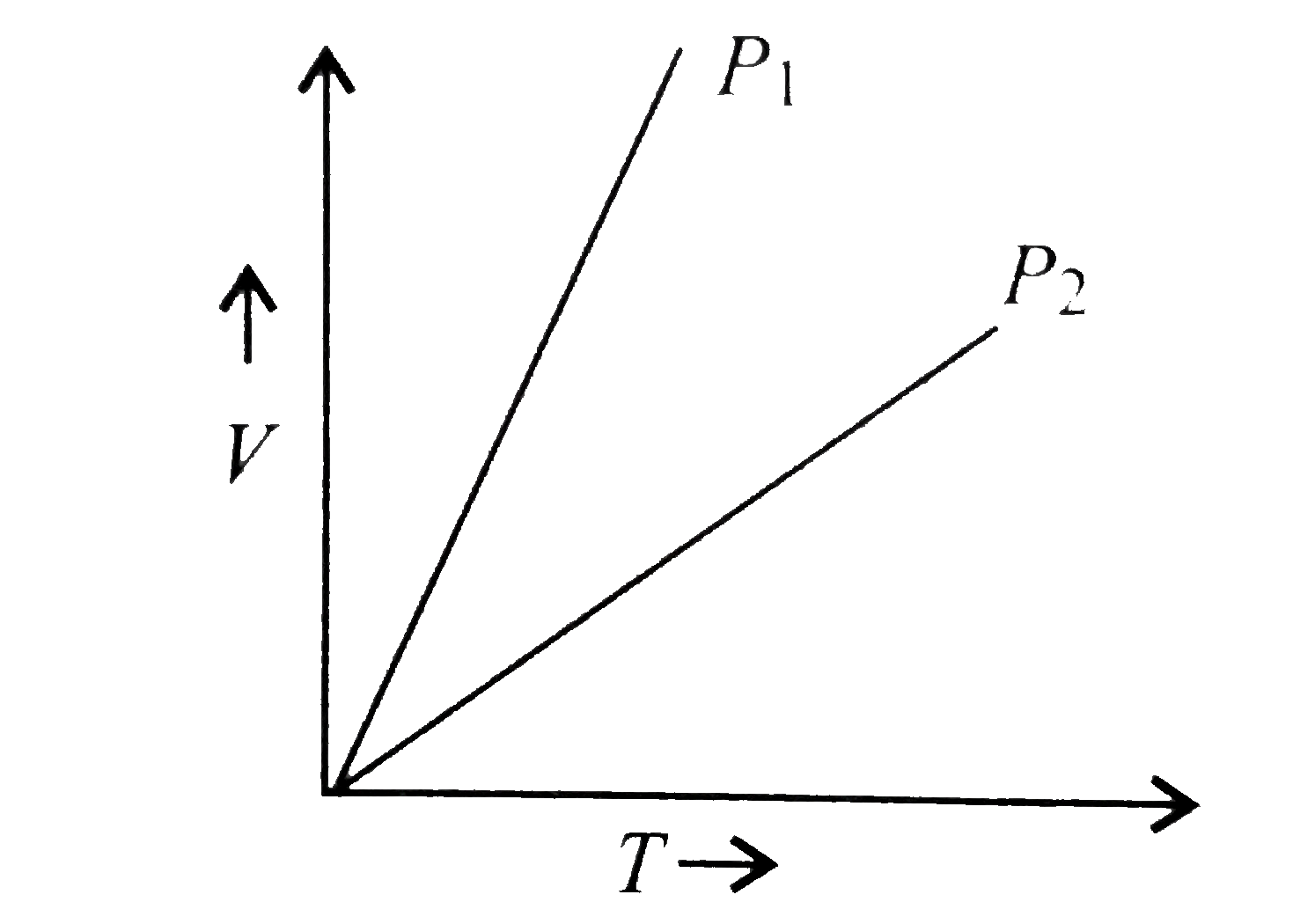
For an ideal gas V−T curves at constant pressure P1 & P2 are shown in figure, from the figure
_E01_061_Q01.png)
View SolutionIn the given (V-T) diagram, what is the relation between pressure P_(1) and P_(2) ?
View SolutionIn the given (V-T) diagram, what is the relation between pressure P1andP2 ?
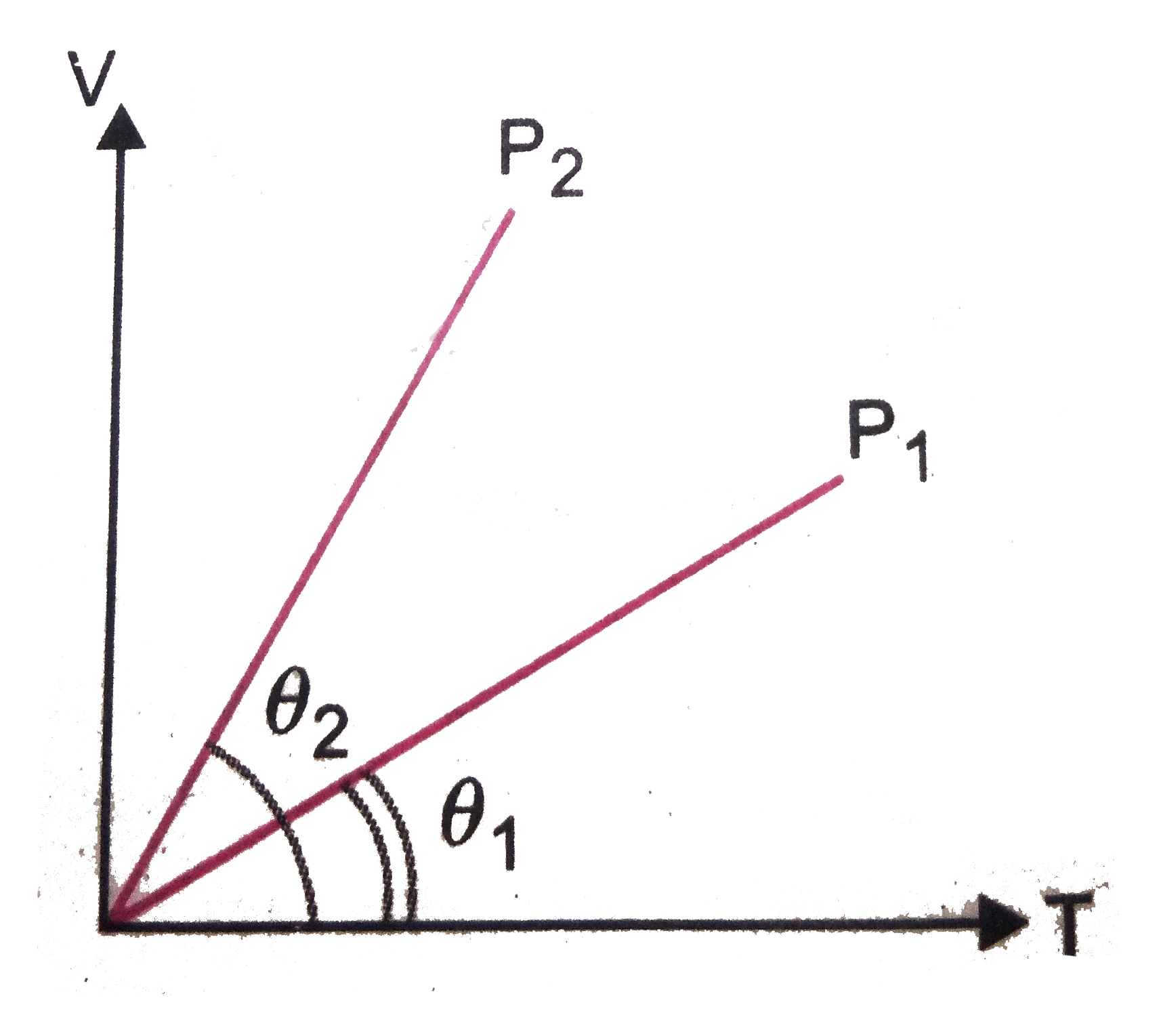
View SolutionV vs T curves at different pressures P1 and P2 for an ideal gas are shown below:
Which one of the following is correct?
View SolutionFor V versus T curves at constant pressure P_(1) and P_(2) for an ideal gas are shown in the figure given below
View SolutionIn the given (V-T) diagram, what is the relation between pressure P_(1) and P_(2) ?
View SolutionV vs T curves at constant pressure P_(1) and P_(2) for an ideal gas are shown below Which is correct ?
View SolutionFor the homogenous gaseous reaction A→3B, if pressure after time t was Pt and after completion of reaction, pressure was P∞ then select correct relation
a) k=1tln(P∞3(P∞−Pt))
b) k=1tln(2P∞(P∞−PT))
c) k=1tln(3P∞2P∞−Pt)
d) k=1tln(P∞3(P∞−PT))
View Solution

