At constant pressure, the value of V/T for different quantities of an ideal gas will be different. Is this statement true or false?Solution in Bengali
Equation of state for n moles of an ideal gas is: PV=nRT
Therefore, VT=nRP
Whe the pressure remains constant,
V/T∝n [∵R=constant]
Thus, at constant pressure, V/T for different quantities of an ideal gas will be different.
Similar Questions
किसी ताप T पर आदर्श गैस के लिए निम्न में से सत्य कथन है
View SolutionA plot of volume (V) versus temperature (T) for a gas at constant pressure is a straight line passing through the origin. The plots at different values of pressure are shown in figure. Which of the following order of pressure is correct for this gas ?
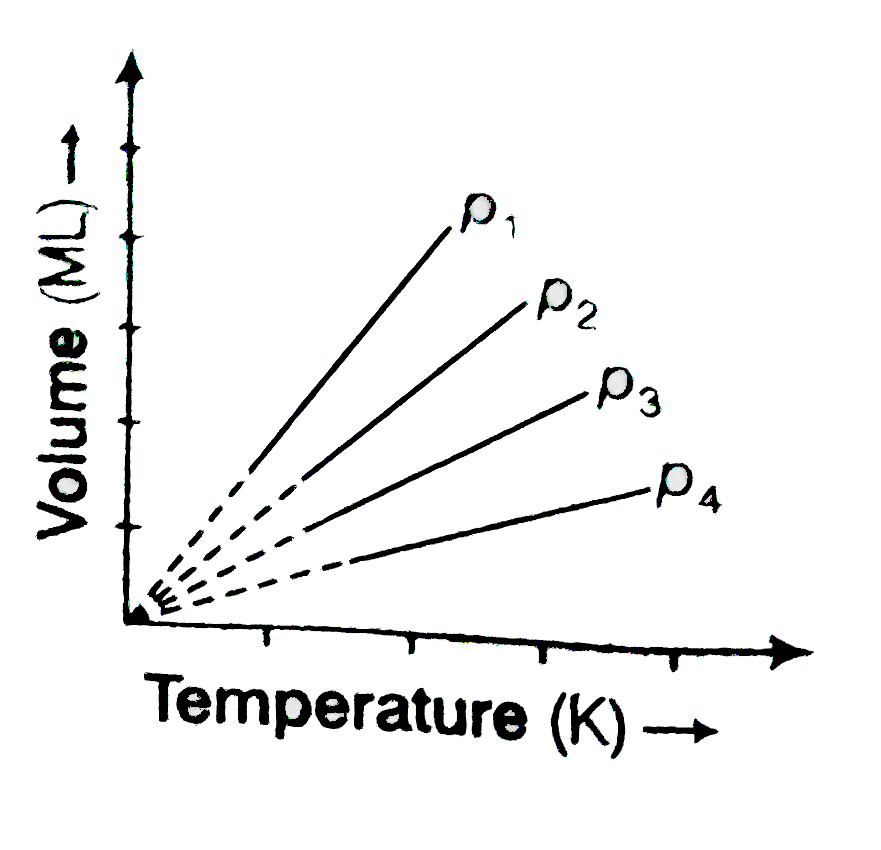
View SolutionThe difference between volume and pressure coefficients of an ideal gas is
View SolutionA plot of volume (V) versus temperature (T) for a gas at constant pressure is a straight line passing through the origin . The plots at different values of pressure are shown in figure .
Which of the following order of pressure is correct for this gas ?
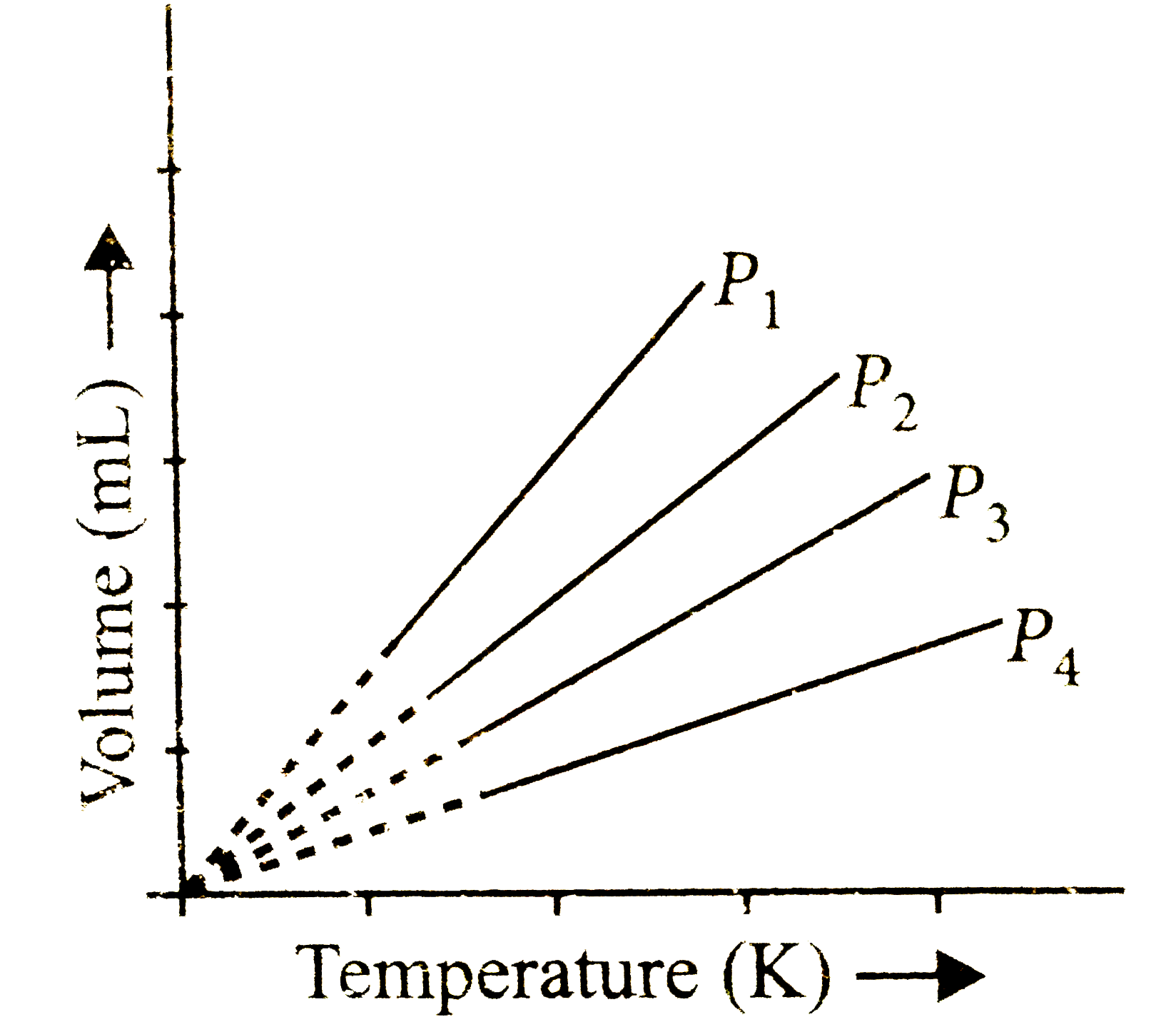
View SolutionTwo different at constant. The relationship between volume V and the pressure P at a given temperature of same ideal gas are shown for masses m_1 and m_2 of the gas respectively. Then
View Solutionस्थिर दाब पर एक गैस के लिए आयतन (V) और ताप (T) के मध्य खींचा गया आरेख मूल बिंदु से होकर जाने वाली सीधी रेखा होती है| दाब के भिन्न मानों पर आरेख में दिखाए हैं| इस गैस के लिए निम्नलिखित में से दाब का कौन-सा क्रम सही है?
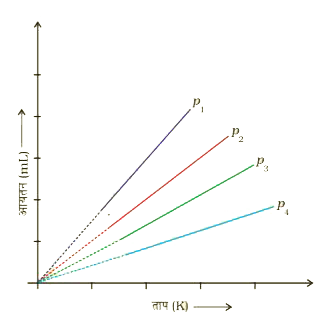
View Solutionकिसी ताप T पर आदर्श गैस के लिए में से असत्य कथन है
View Solutionनियत दाब पर आदर्श गैस के लिए (VT) तथा (1V) के मध्य सही आरेख होगा -
View Solution
