Why is the PV vs P plot for an ideal gas at a given temperature parallel to P axis?Solution in Bengali
Video Solution
Text Solution
At a given temperature for a given amoun of gas PV is always is constant, irrespective of the value of P.
|
Similar Questions
The figure shows the plot of PVnT vs P, for oxygen gas at two different temperatures then
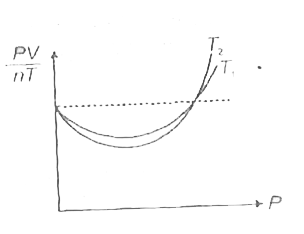
View SolutionFor a certain gas which deviates a little from ideal behaviour, a plot between P/p vs P was found to be non- linear . The intercept on y-axis will be :
View SolutionWhy real gas shows deviation of ideal gas at fixed temperature in geaph of pV → p and p→V.
View SolutionBased upon Boyle's law draw the plot of P vs V and also PV vs P.
View SolutionThe graph of PV vs P for a gas is
View SolutionThe graph between PV vs P at constant temperature is linear parallel to the pressure axis.
View SolutionThe plot of PV vs P at particular temperature is called isovbar.
View SolutionThe figure shows the plot of PVnT vs P, for oxygen gas at two different temperatures then

View Solution

