At room temperature 2NO+O2→2NO2→N2O4 reaction proceeds near to completion. The dimer, N2O4, solidified at 262K. A 250 mL flask and a 100 mL flask are separated by a stop-cock. At 300K, nitric oxide in the longer flask exerts a presure of 1.053 atm and and the smaller one contains oxygen at 0789 atm. the gases are mixed by opening the stopcock and after the end off the reaction the flasks are cooled to 220K. Neglecting vapour pressure of the dimer, find out pressure and composition of the gas remaining at 220 K (assume that the gases behave ideally).Solution in Bengali
Number of moles of NO gas is 250 mL flask,
PVRT=1.053×0.250.0821×300=0.01 mol
and that of O2 in 100mL flask,
=PVRT=0.789×0.10.0821×300=3.2×10−3 mol.
reaction: 2NO+O2→2NO2
Thus 1 mol of O2 gas completely reacts with 2 mol of NO gas.
therefore, 3.2×10−3 mol of O2 gas will react with 2×3.2×10−3=6.4×10−3mol of NO.
After the completion of reaction number of moles of NO left in the reaction system=(0.01−6.4×10−3)
=3.6×10−3mol
Total volume of the reaction system=(0.25+0.1)L=0.35L
If the pressure of NO gas left in the reaction system be P, then P×0.35=3.6×10−3×0.0821x220
∴P=0.185 atm
∴After the reaction, the pressure of NO gas is 0.185 atm.
Similar Questions
Two flasks A and B of 500 mL each are respectivelly filled with O2 and SO2 at 300 K and 1 atm. Pressure . The flasks will contain:
View SolutionA gaseous mixture containing 0.35g of N2 and 5600 ml of O2 at STP is kept in a 5 litres flask at 300K. The total pressure of the gaseous mixture is
View SolutionTwo flasks A and B of 500 mL each are respectivelly filled with O_(2)" and "SO_(2) at 300 K and 1 atm. Pressure . The flasks will contain:
View SolutionAt room temperature following traction goes to completion
2AB(g)+B2(g)→2AB2(s)
AB2 is solid with negligble vapour pressure below 0∘C. At 300 K, the AB in the smaller flask exerts a pressure of 3 atm and in the larger flask B2 exerts a pressure of 1 atm at 400 K when they are separated out by a close valve, The gases are mixed by opening the stop cock and after the end of the reaction the flask are cooled to 250 K
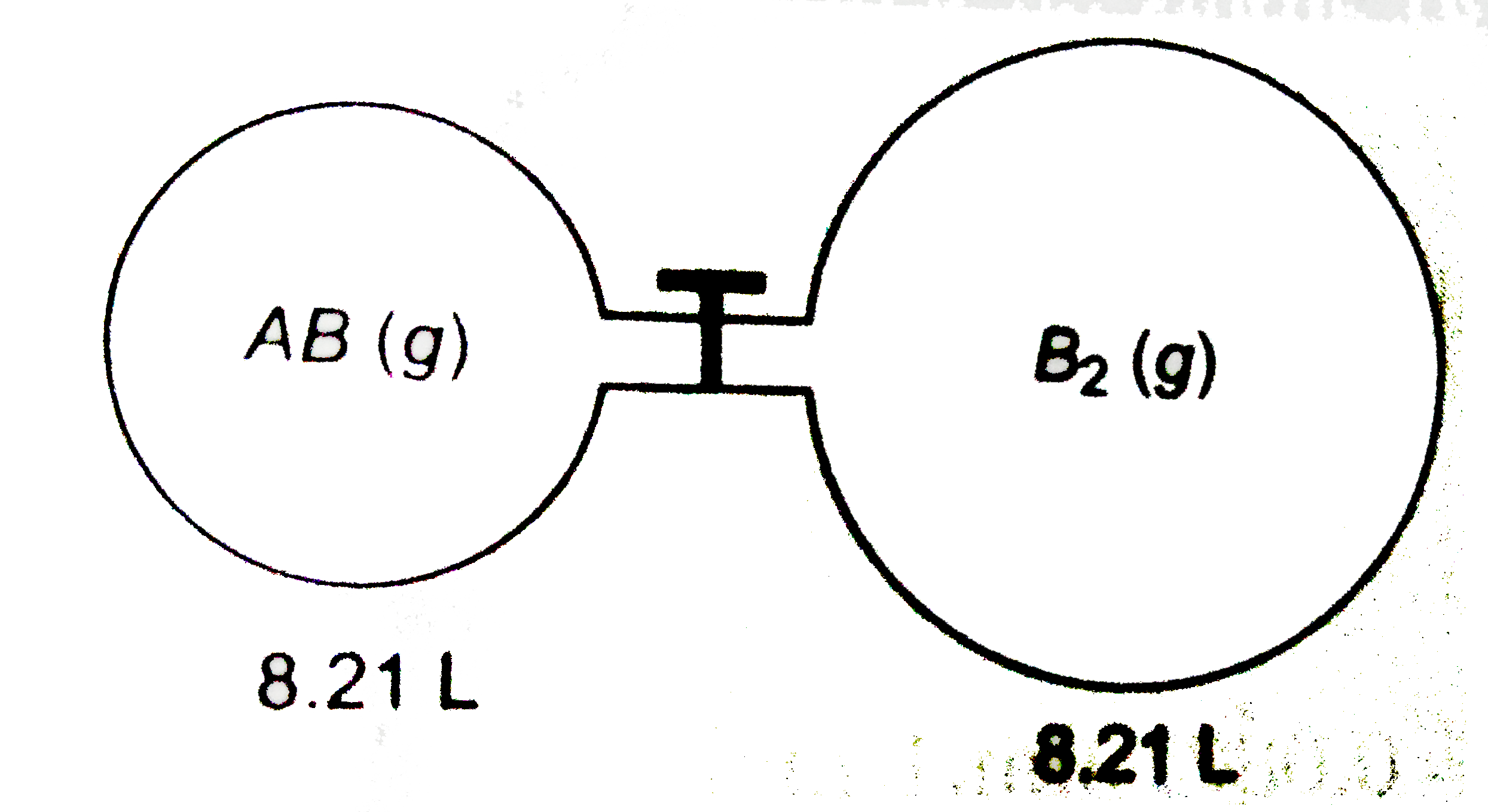
The final pressure is :
View SolutionAt room temperature the following reactions proceed nearly to completion: 2NO + O_2 to 2NO_2 to N_2O_4 The dimer, N_2O_4 solidifies at 262 K. A 250-ml flask and a 100-ml flask are separated by a stopcock. At 300 K the nitric oxide in the larger flask exerts a pressure of 1.053 atm and the smaller one contains oxygen at 0.789 atm. The gases are mixed by opening the stopcock and after the end of the reaction the flasks are cooled to 220 K. Neglecting the vapour pressure of the dimer, find out the pressure and composition of the gas remaining at 220 K. Assume the gases to behave ideally.
View SolutionAt room temperature, the following reaction proceeds nearly to completion: 2NO+O_(2)to2NO_(2)toN_(2)O_(4) The dimer, N_(2)O_(4) , solidfies at 262 K . A 250 mL flask and a 100 mL flask are separated by a stopcock. At 300 K , the nitric oxide in the larger flask exerts a pressure of 1.053 atm and the smaller one contains oxygen at 0.789 atm . The gase are mixed by opening the stopcock and after the end of the reaction the flasks are cooled to 220 K . Neglecting the vapour pressure of the dimer, find out the pressure and composition of the gas remaining at 220 K . (Assume the gases to behave ideally)
View SolutionAt room temperature, the following reaction proceeds nearly to completion:
2NO+O2→2NO2→N2O4
The dimer, N2O4, solidfies at 262K. A 250mL flask and a 100mL flask are separated by a stopcock. At 300K, the nitric oxide in the larger flask exerts a pressure of 1.053atm and the smaller one contains oxygen at 0.789atm. The gase are mixed by opening the stopcock and after the end of the reaction the flasks are cooled to 220K. Neglecting the vapour pressure of the dimer, find out the pressure and composition of the gas remaining at 220K. (Assume the gases to behave ideally)
View SolutionAt room temperature following traction goes to completion 2AB(g)+B_(2)(g)rarr2AB_(2)(s) AB_(2) is solid with negligble vapour pressure below 0^(@)C . At 300 K, the AB in the smaller flask exerts a pressure of 3 atm and in the larger flask B_(2) exerts a pressure of 1 atm at 400 K when they are separated out by a close valve, The gases are mixed by opening the stop cock and after the end of the reaction the flask are cooled to 250 K The final pressure is :
View Solution
