Carbon anode in the electrolytic reduction of Al2O3.Solution in Bengali
Step by step video & image solution for Carbon anode in the electrolytic reduction of Al_(2)O_(3). by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
What acts as cathod ein electrolytic reduction of Al_(2)O_(3)?
View SolutionCarbon cannot be used in the reduction of Al_(2)O_(3) because
View SolutionExtraction of Aluminiunt can be understood by : Electrolytic reduction of Al_(2)O_(3) : {:("Electrolyte",:(Al_(2)O_(3)+"Cryolite")),("Cathode",:"Graphite inside the Fe container"),("Anode",:"Graphite rods"):} The purpose of adding cryolite is
View SolutionExtraction of Aluminiunt can be understood by : Electrolytic reduction of Al_(2)O_(3) : {:("Electrolyte",:(Al_(2)O_(3)+"Cryolite")),("Cathode",:"Graphite inside the Fe container"),("Anode",:"Graphite rods"):} Coke powder is spreaded over the molten electrolyte to
View SolutionExtraction of Aluminiunt can be understood by :
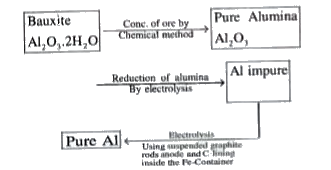
Electrolytic reduction of Al2O3:
Electrolyte:(Al2O3+Cryolite)Cathode:Graphite inside the Fe containerAnode:Graphite rods
The function of fluorur (CaF2) is
View SolutionExtraction of Aluminiunt can be understood by : Electrolytic reduction of Al_(2)O_(3) : {:("Electrolyte",:(Al_(2)O_(3)+"Cryolite")),("Cathode",:"Graphite inside the Fe container"),("Anode",:"Graphite rods"):} The molten electrolytes contains Na^(+),Ca^(2+), Ca^(2+) but only Al get deposited at cathode because :
View SolutionAl2O3 का अपचयन कार्बन के द्वारा सम्भव नहीं हैं क्योंकि
View Solution

