Complex of HgCo.4SCN has two isomers XandY then, which of the following option is incorrect ?
A
X and Y are different in structure
B
Oxidation of HgandCo are same in both the complex
C
X has △0<P
D
both have same hybridisation.
Video Solution
Text Solution
The correct Answer is:D
+2Hg[+2Co(SCN)4],+2Hg[+2Co(NCS)4],SCN−is a W.F.L. while NCS−is a S.F.L.
|
Similar Questions
Knowledge Check
Which of the following options is incorrect?
ApKa1 cis but-2-ene dioic acid gt trans but-2-ene dioic acidBdipole moment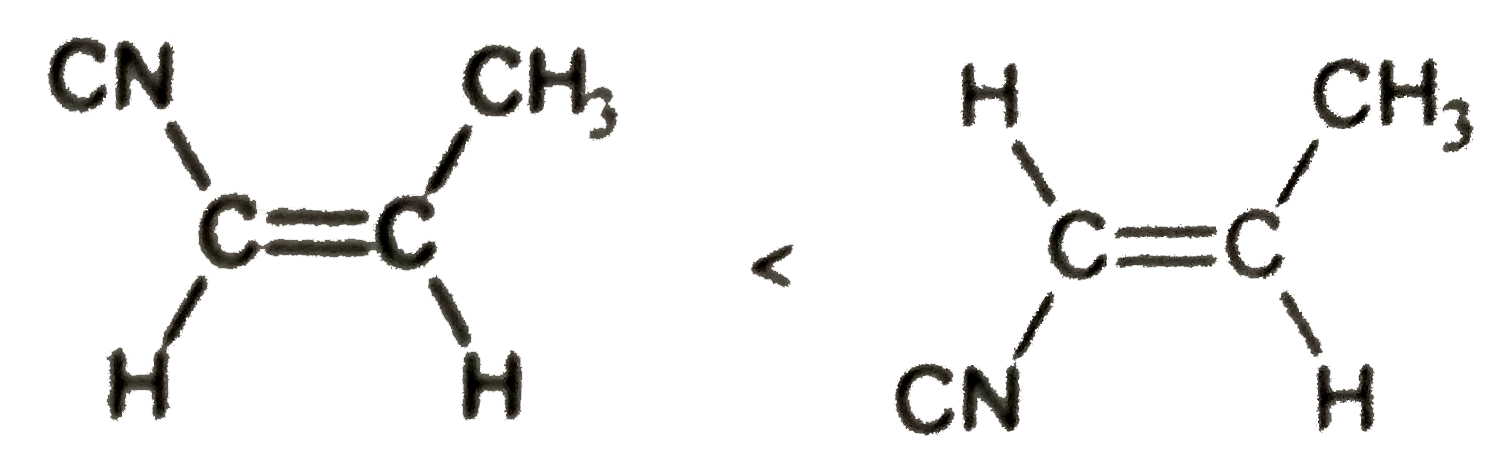 CStability
CStability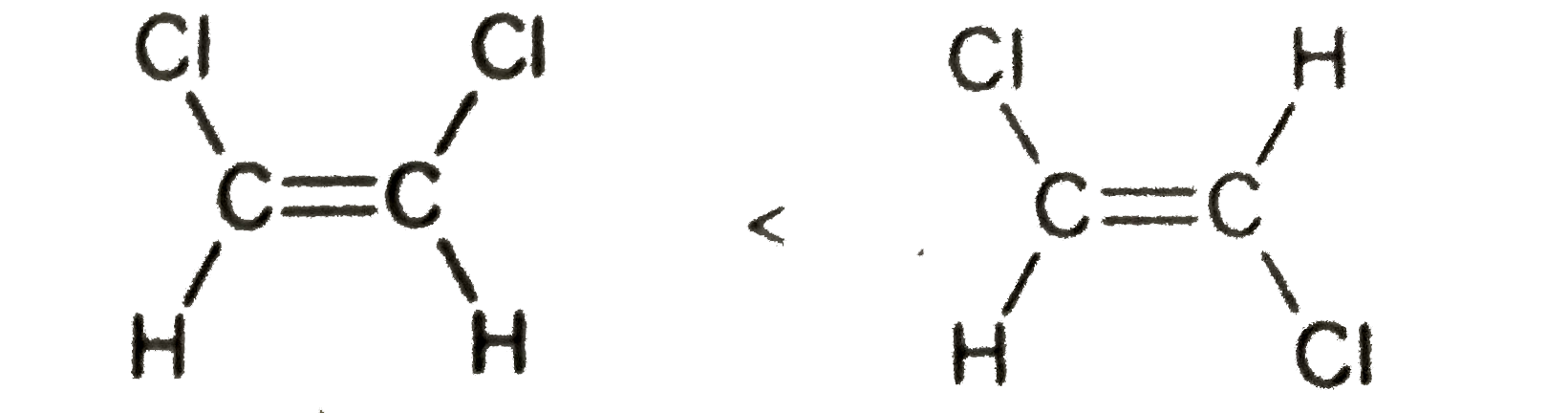 DBasicity
DBasicity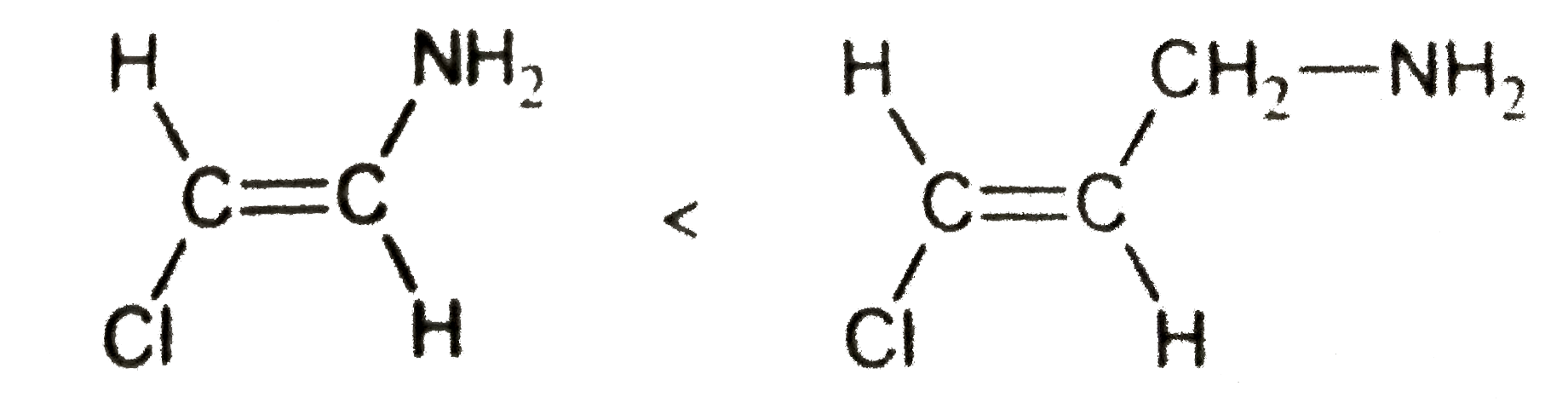
Which one of the following option is incorrect ?
ApKa1 cis but-2-ene dioic acid gt trans but-2-ene dioic acidBdipole moment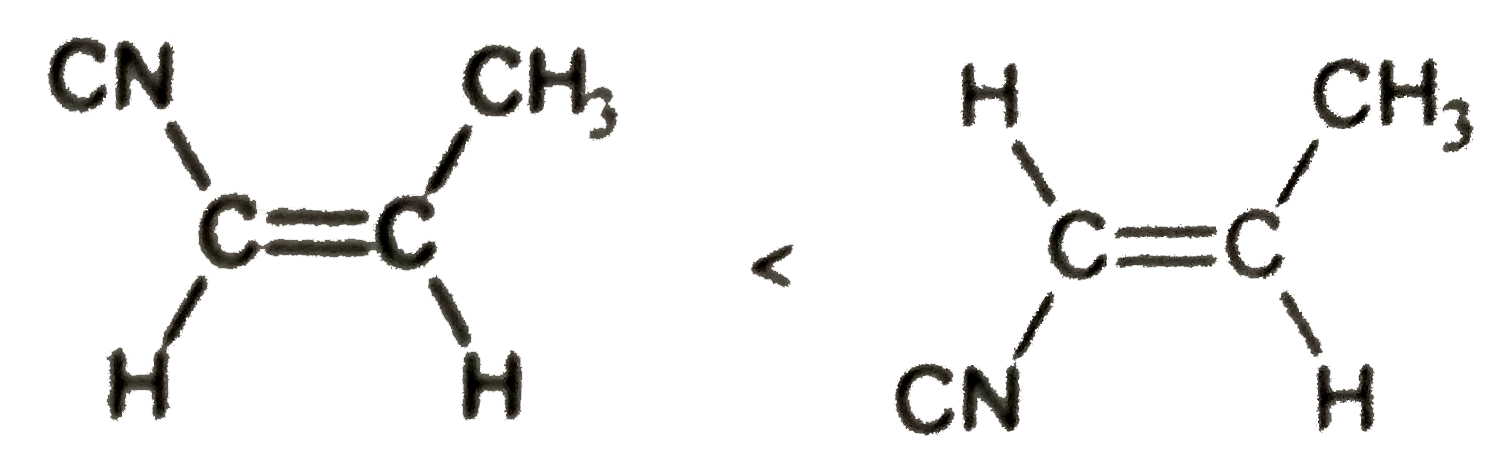 CStability
CStability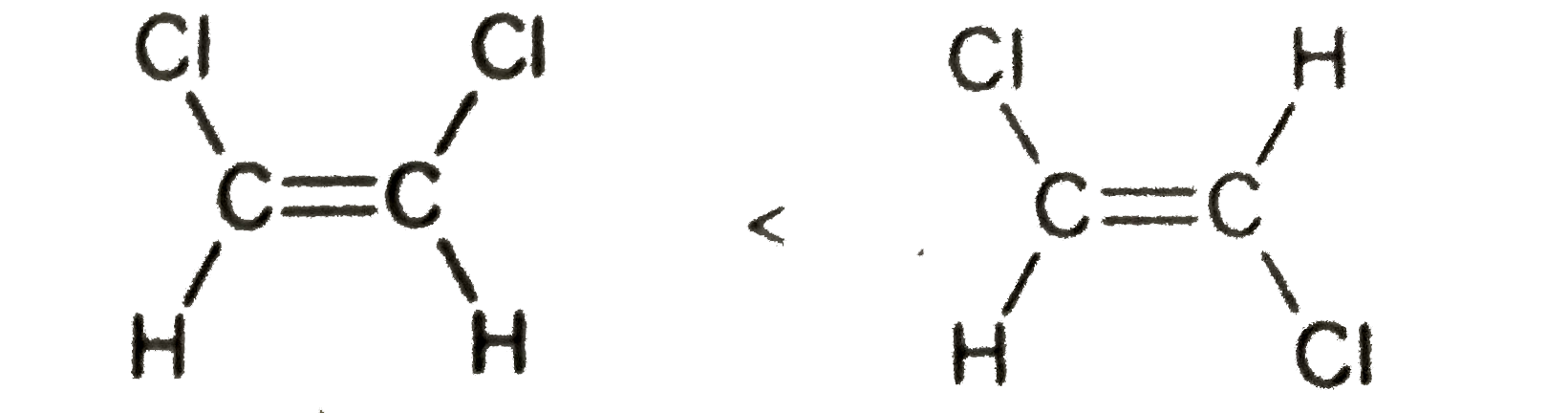 DBasicity
DBasicity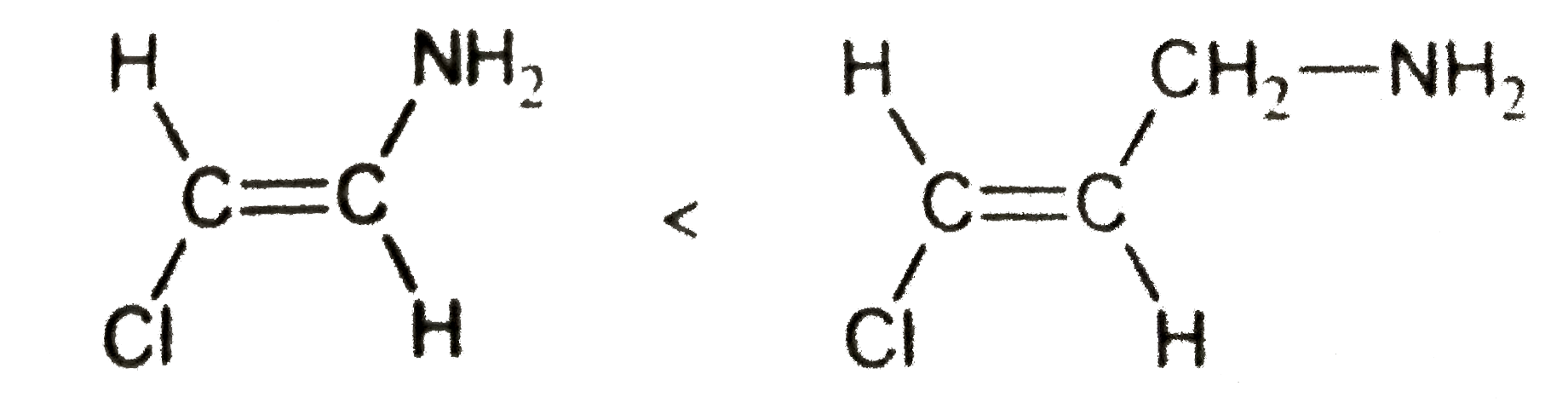
Which of the following option(s) is/are incorrect?
A∫π/80∣∣∣sin(8nx)+cos(8nx)x∣∣∣dx<2√2π(1+12+13+………+1n)B2In3<∫103x1/3dx<∫104x1/3dxC∫π/60√sinx(8−3√sinx)dx>8π9D14<∫π/3π/4tanxxdx<1√3
Complex of HgC⊙4SCN has two isomers X>Y then, which of the following option is incorrect ?
View SolutionWhich of the following options are incorrect.
View SolutionWhich one of the following options is incorrect?
View SolutionWhich of the following is incorrect option
View SolutionWhich of the following has an optical isomer ?
View Solution
