The complex [Fe(H2O)5NO]2+ is formed in the 'brown ring test' for nitrates. Choose the incorrect statement for the complex.
Its magnetic moment is approximately 3.9 BM
The oxidation state of iron is +1
The hybridization of central metal ion is sp3d2
The brown colour of the ring is due to d−d transition
The correct Answer is:D
[+1Fe(H2O)5+NO]2+
sp3d2,d7 config.
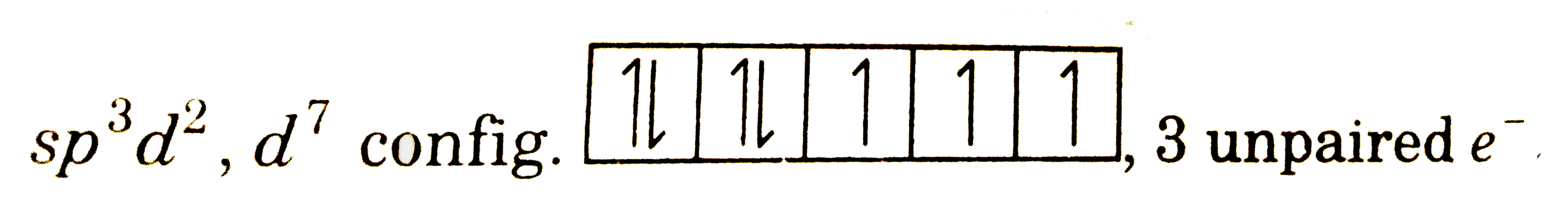
3 unpaired e−
Similar Questions
The brown ring test for nitrates depends on
View SolutionBrown ring test for nitrates depends on
I. the ability of Fe2+ to reduce nitrates to nitric oxide,
II. Its reaction with Fe2+ to form a brown coloured complex.
Which of the above statements regarding brown test for nitrates is/are true?
View Solution
Knowledge Check
The complex [Fe(H2O)5NO]2+ is formed in Brown ring test for nitrates select correct statement for the complex ?
AHybridisation of iron is sp3d2BIron and nitrosyl has +1 oxidation stateCIt has magnetic moment of 3.87 B.M.DAll are correctThe brown ring test for nitrate employs
ABarium chlorideBFerrous sulphatesCNitric acidDnone of the aboveIn the complex ion [Fe(H2O)5NO]2+
AFe is in the =2 oxidation state and NO coordinates as NO+BFe is in the +3 oxidation state and NO coordinates as NO−CFe is in the +2 oxidation state and NO coordinates as neutral NO (nitrosyl) radicalDFe is in the +1 oxidation state and NO coordinates as NO+ (nitrosonium ion)
The brown ring test for nitrates depends on
View SolutionThe brown ring test for nitrates depends on
View SolutionThe brown ring test for nitrates depends on
View SolutionThe complex [Fe(H2O)5NO]2+ is formed in the brown ring test for nitrates when freshly prepared FeSO4 solution is added to aqueous solution of NO−3 followed by addition of conc.H2SO4 . Select the correct statement about this complex.
View SolutionThe complex [Fe(H2O)5NO]2+ is formed in the brown ring test for nitrates when freshly prepared FeSO4 solution is added to aqueous solution of NO3 followed by addition of cone. H2SO4. Select correct statement about this complex.
View Solution

