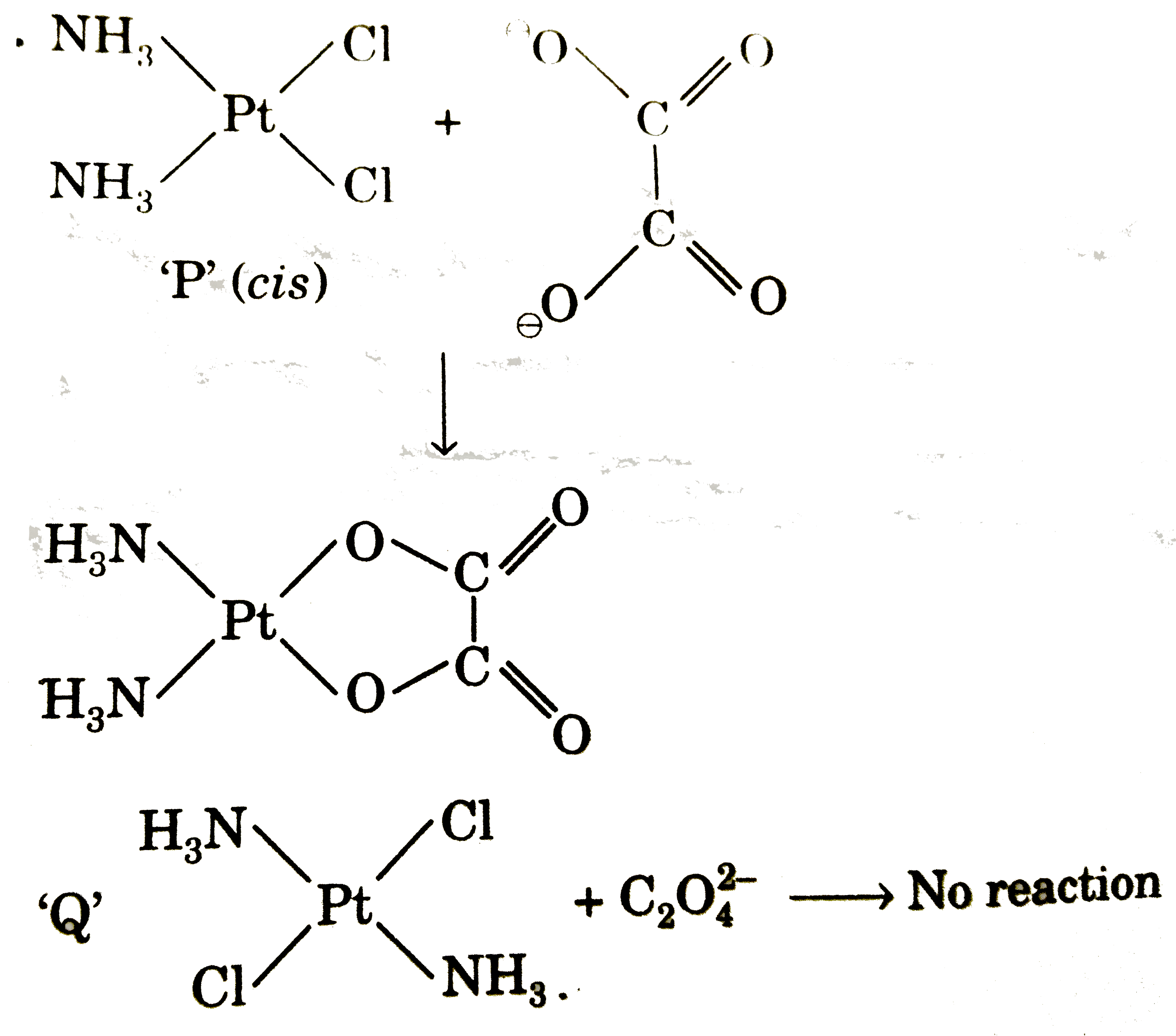
On treatment of [Pt(NH3)4]2+ with concentrated HCI, two compounds (P) and (Q) having the same formula, [Pt(NH3)2CL2] are obtained, (P) can be converted into (Q) by boiling with dilute HCI. A soution of (P) reacts with oxalic acid to form [Pt(NH3)2(C2O4)] whereas (Q) does not react. Point out the correct statement of the following :
(P) cis, (Q) trans, both tetrahedral
(P) cis, (Q) trans, both square planar
(P) trains, (Q) cis, both tetrahedral
(P) trans, (Q) cis, both square planar
The correct Answer is:B
Topper's Solved these Questions
- COORDINATION COMPOUNDSBook:GRB PUBLICATIONChapter:COORDINATION COMPOUNDSExercise:G. Organometallic Compounds, Synergic Bonding
Explore 52 Videos
- COORDINATION COMPOUNDSBook:GRB PUBLICATIONChapter:COORDINATION COMPOUNDSExercise:REASONING TYPE
Explore 59 Videos
- COORDINATION COMPOUNDSBook:GRB PUBLICATIONChapter:COORDINATION COMPOUNDSExercise:E. Stability, Colour and Magnetic Properties of Complexes
Explore 60 Videos
- CHEMICAL KINETICSBook:GRB PUBLICATIONChapter:CHEMICAL KINETICSExercise:Subjective Type
Explore 63 Videos
- D-BLOCK ELEMENTSBook:GRB PUBLICATIONChapter:D-BLOCK ELEMENTSExercise:Subjective Type
Explore 18 Videos
Similar Questions
When [Ni(NH3)4]2+ is treated with conc HCI, two compounds having the formula Ni(NH3)(2)CI2 are formed. A solution of I reacts with oxalic acid to form Ni(NH3)2(C2O4). II does not react with oxalic acid. Deduce the configuration of I and II and the geometry of Ni (II) complexes .
View SolutionThe correct IUPAC name of [Pt(NH3)2Cl2] is
View Solution
Knowledge Check
The compound [Pt(NH3)2Cl2] can exhibit
Alinkage isomerismBoptical isomerismCgeometrical isomersimDionisation isomerism[Pt(NH3)4]CL2 is
ApyramidalBpentagonalCtetrahedralDsquare planarThe EAN of Pt in [Pt(NH3)4]2+
A33B34C35D36
When [Ni(NH3)4]2+ is treated with conc HCI two compounds having the formula Ni(NH3)CI2 (designated as I and II are formed) I can be converted into II ny boiling in dil HCI A solution of I reacts with oxalic acid to form Ni(NH3)2(C2O4) II does not react with oxalic acid Deduce the configuration Of I and II and the geometry of Ni (II) complexes .
View SolutionIUPAC name of [Pt(NH3)2Cl(NO2)] is:
View SolutionIUPAC name of [Pt(NH3)2Cl(NO2)] is
View SolutionIUPAC name of [Pt(NH3)2Cl(NO2)] is
View SolutionThe correct name of ∣∣Pt(NH3)4Cl2∣∣|PtCl4| is
View Solution