Which of the following is true about the complex [PtCl2(NH3)OH2] ? [Atomic number of Pt=78]
(P) It will have two geometrical isomeric forms, cis and trans
(Q) The hybridisation state of Pt(II) is sp2
(R ) It is a square planar complex
(S) It is a diamagnetic complex
(T) It can show hydrate isomerism
(U) It is tetrahedral complex
A
P, R, S
B
Q, S, T
C
Q, T, U
D
P,T,U
Video Solution
Text Solution
The correct Answer is:A
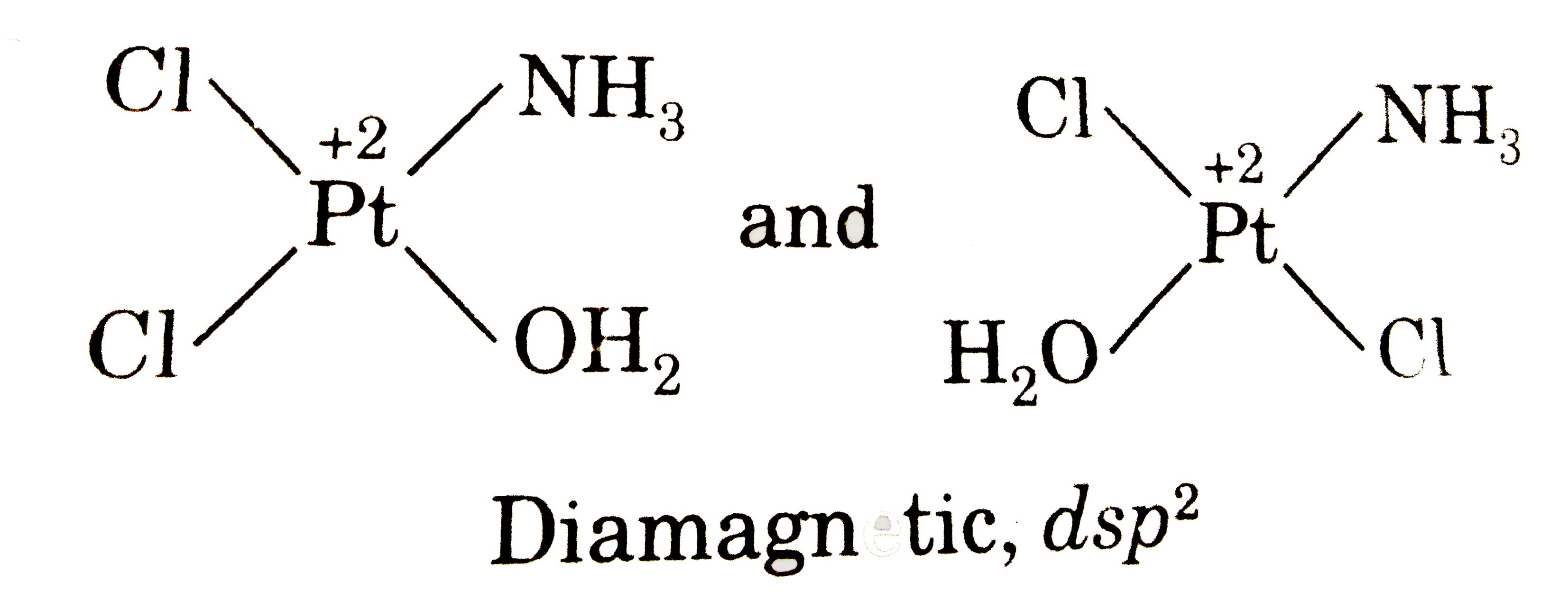 Diamagnetic , dsp2
Diamagnetic , dsp2
|
Similar Questions
Knowledge Check
Which of the following is true about the complex [PtCl2(NH3)(OH2)]? [ Atomic number of Pt=78]
(i) It will have two geometrical isomeric forms, cis and trans.
(ii) The hybridisation state of Pt(II) is sp3.
(iii) It is a square planar complex. (iv) It is a diamagnetic complex.
(v) It can show hydrate isomerism. (vi) It is a tetrahedral complex.A(i),(iii),(iv)B(ii),(iv),(v)C(ii),(v),(vi)D(i),(v),(vi)Which of the following is true about the complex [PtCl2(H2O)(NH3)]?
AIt exhibits geomettrical isomerismBIt is paramagnetic complexCits geometry is tetrahedronDplatinum is sp3 hybridisedWhich of the following is true about the complex [PtCl2(H2O)(NH3)]?
AIt exhibits geomettrical isomerismBIt is paramagnetic complexCits geometry is tetrahedronDplatinum is sp3 hybridised
Which of the followng isomerism is /are shown by the complex [CoCl2(OH)2(NH3)2]Br ?
View SolutionWhich of the following statements is not true about the complex ion [Pt(en)2Cl2]2+?
View SolutionWhich of the followng isomerism is /are shown by the complex [CoCl2(OH)2(NH3)2]Br ?
View SolutionWhich of the following isomerism is/are shown by the complex [CoCl2(OH2)2(NH3)2]Br?
View SolutionWhich of the following isomerism is/are not shown by the complex [Co(Cl2(OH)2(NH3)2]Br?
View Solution
