Which of the following statements is/are correct ?
The stability constant of [Co(NH3)6]3+ is greater than that of [Co(NH3)6]2+
Among F−,Cl−,Br− and I−,F− forms more stable complexes due to small size.
[Cu(NH3)4]2+ is thermodynamically more stable than [Zn(NH3)4]2+
Among [Fe(CN)6]3−,[Fe(H2O)6]2+ and [Fe(en)3]3+,[Fe(CN)6]3− is most stable.
The correct Answer is:A, C
(c ) According to Irving Williams order, stability of +2 oxidation state for 3d transition series follows :
MnII<FeII<CoII<NiII<CuII>ZnII
(d) [Fe(en)3]3+ is more stable due to chelation.
Topper's Solved these Questions
- COORDINATION COMPOUNDSBook:GRB PUBLICATIONChapter:COORDINATION COMPOUNDSExercise:COMPREHENSION 1
Explore 3 Videos
- COORDINATION COMPOUNDSBook:GRB PUBLICATIONChapter:COORDINATION COMPOUNDSExercise:COMPREHENSION 2
Explore 3 Videos
- COORDINATION COMPOUNDSBook:GRB PUBLICATIONChapter:COORDINATION COMPOUNDSExercise:REASONING TYPE
Explore 59 Videos
- CHEMICAL KINETICSBook:GRB PUBLICATIONChapter:CHEMICAL KINETICSExercise:Subjective Type
Explore 63 Videos
- D-BLOCK ELEMENTSBook:GRB PUBLICATIONChapter:D-BLOCK ELEMENTSExercise:Subjective Type
Explore 18 Videos
Similar Questions
18 electron rule: Valence shell of a transition metal consists of nine valence orbitals of (n-1) d, ns and np sub-shells, which can accommodate 18 electrons. When a metal complex has 18 valence electrons, it is said to have achieved the same electronic cofiguration as the noble gas at the end of the period. The rule and its exception are similar to the application of the octet rule of main group elements. The rule is not helpful for complexes of the s-block metals, the lanthanides or actinides. Effective atomic number is the total number of electrons possesed by the central possesed by the central metal atom/ion including the electrons contributed by the ligands. It is obvious that if a central transition metal atom/ion achieves 18 electrons in its valence shell, it attains the nearest noble gas cofiguration, which is known as effective atomic numbr (EAN) rule.
[Atomic number :Cr=24, Fe=26, Cu=29, Ni=28, Rh=45]
Which of the following does not obey EAN rule or 18 electron rule ?
View Solution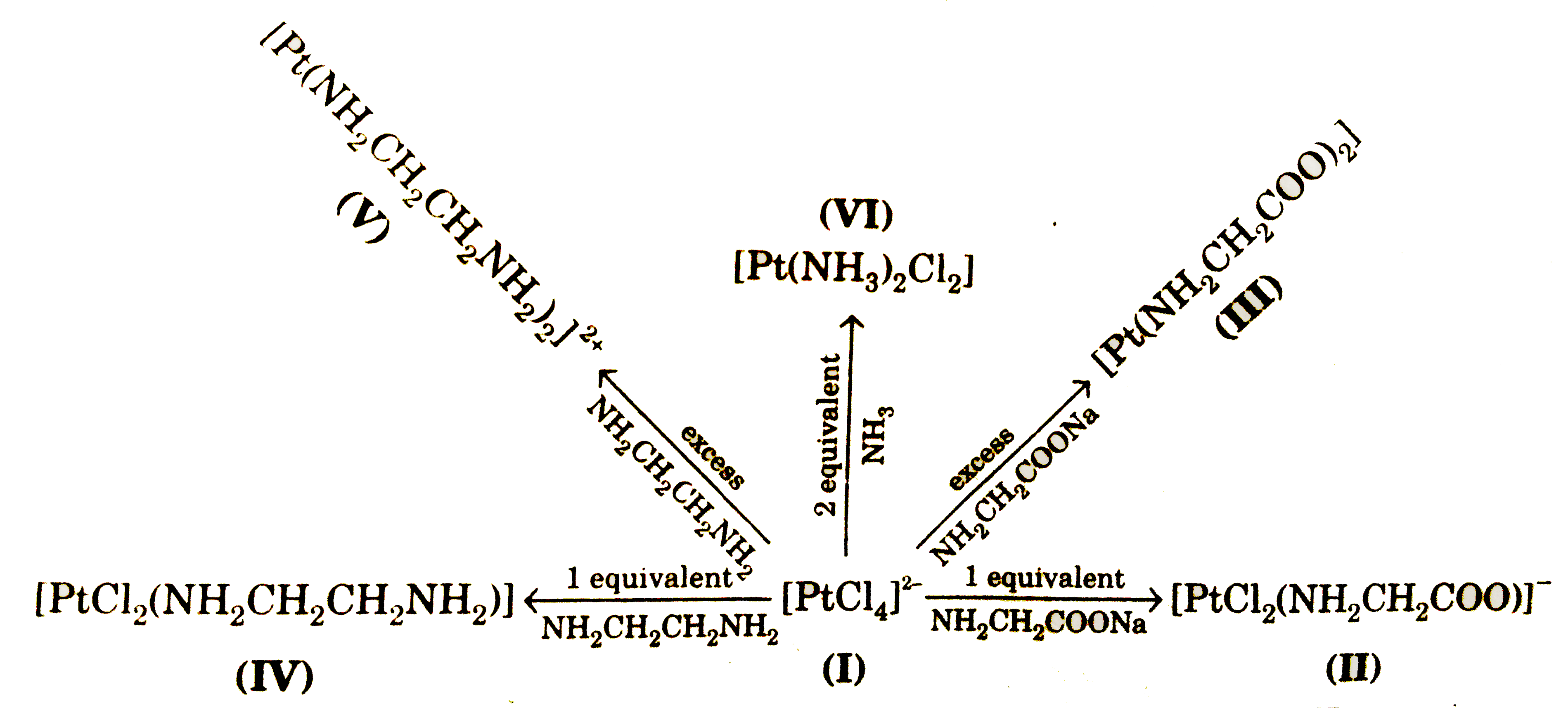
All these complexes are square planar about PtII.Square planar complexes show geometrical isomerism provided ligands are proper. A bidentate ligand (like ethylenediamine, glycinato) does not attach itself in anti-position. If the ligands present in square planar complexes do not contain chiral or asymmetric center, such complexes do not show optical isomerism due to presence of plane of symmetry.
Optical isomerism is shown by :
View SolutionStatement-1: In [Co(en)3]Cl3, the coordination number of Co3+ ion is 3.
Statement-2: 'en' is a bidentate ligand.
View SolutionCalculate the specific activity of a radioactive substance .25098 Cf if its half life is 6.93 min . Express your answer in terms of 1016 dps.
(Use:NA=6×1023)
View SolutionNumber of halide ions among (F−,Cl−,Br−,I−) which change their oxidation number on heating with MnO2+conc.H2CO4
View SolutionWhich of the following statements is/are correct?
View SolutionWhich of the following statements are correct statements ?
View SolutionWhich of the following statements is/are correct?
View SolutionWhich of the following statements is/are correct ?
View SolutionWhich of the following is/are correct statement/statements ?
View SolutionWhich of the following statement is/are correct ?
View Solutionनिम्नलिखित कथनों में कौन-सा कथन सही नहीं है-
View SolutionWhich of the following statements is // are correct
View SolutionWhich of the following statements correct?
View SolutionWhich of the following statements is correct:
View SolutionWhich of the following statements is correct-
View Solution

