Select the correct statements :
The smaller the ligand, the closer it comes to the metal ion and thus the greater the repulsion between ligand and the electrons present in the d-orbital of central atom that is why I−<Br−<CI−<F− are present in spectrochemical series as order.
Aminoethanethiolate complex of Pt(II),[Pt(NH2CH2CH2S)2] has no enantiomers but has geometrical isomers while aminoethanethiolate complex of coba<(II),[Co(NH2CH2CH2S)2] has no enantiomers and no geometrical isomers.
[Ni(H2O)6]2+ is green,[Ni(NH3)6]2+ is deep blue and [Ni(CN)4]2− is yellow because [Ni(H2O)6]2+ has absorbed red colour , [Ni(NH3)6]2+ has absorbed orange colour and [Ni(CN)4]2− has absorbed violet indigo colour.
[Fe(C2O4)3]3− complex ion has d2sp3 hybridisation, is inner orbital complex and low spin.
The correct Answer is:A, C
[F(C2O4)3]3− is outer orbital.
Topper's Solved these Questions
- COORDINATION COMPOUNDSBOOK - GRB PUBLICATIONCHAPTER - COORDINATION COMPOUNDSEXERCISE - COMPREHENSION 1
3 Videos
- COORDINATION COMPOUNDSBOOK - GRB PUBLICATIONCHAPTER - COORDINATION COMPOUNDSEXERCISE - COMPREHENSION 2
3 Videos
- COORDINATION COMPOUNDSBOOK - GRB PUBLICATIONCHAPTER - COORDINATION COMPOUNDSEXERCISE - REASONING TYPE
59 Videos
- CHEMICAL KINETICSBOOK - GRB PUBLICATIONCHAPTER - CHEMICAL KINETICSEXERCISE - Subjective Type
63 Videos
- D-BLOCK ELEMENTSBOOK - GRB PUBLICATIONCHAPTER - D-BLOCK ELEMENTSEXERCISE - Subjective Type
18 Videos
Similar Questions
Knowledge Check
Select the correct statements
AH+ can exist as H9O+4 in waterBElectrolysis of fused sodium hydride produces H2 at the anodeCHydride ion is larger that any of the halide ions except iodide ionDAll are correctSelect the correct statement(s) :
AClay and lime on strong heating produces a fused mass known as 'cement clinkers'BMelting point and boiling point of II A group elements are higher than that of corresponding I A group elementsCZeolite method is used to remove permanent hardness of waterD'Ba' is soluble in both water and liquid NH3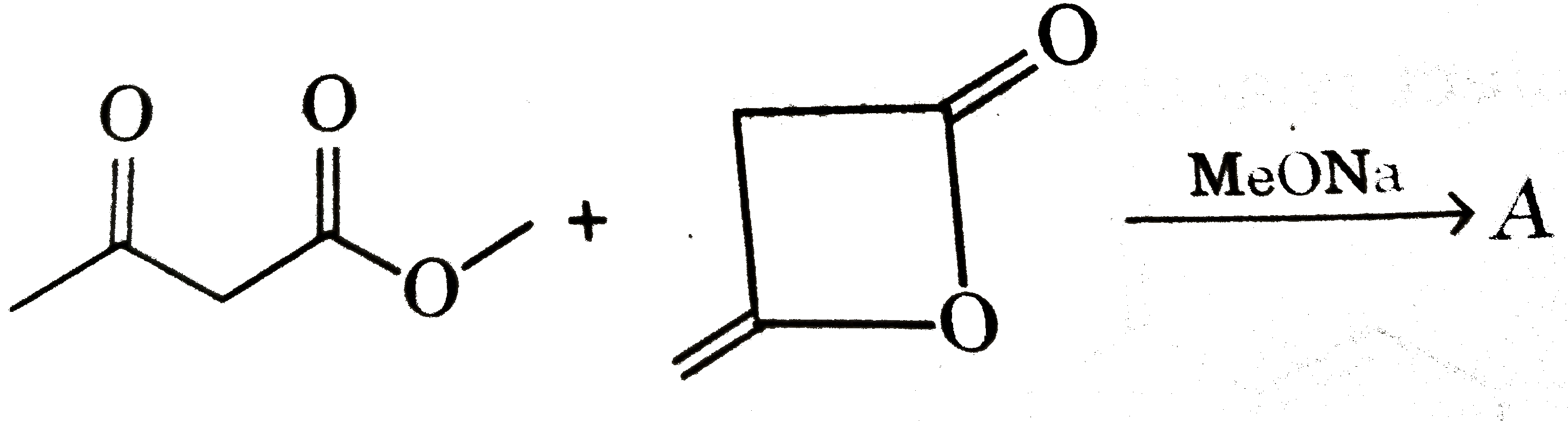
Select the correct statement(s) :A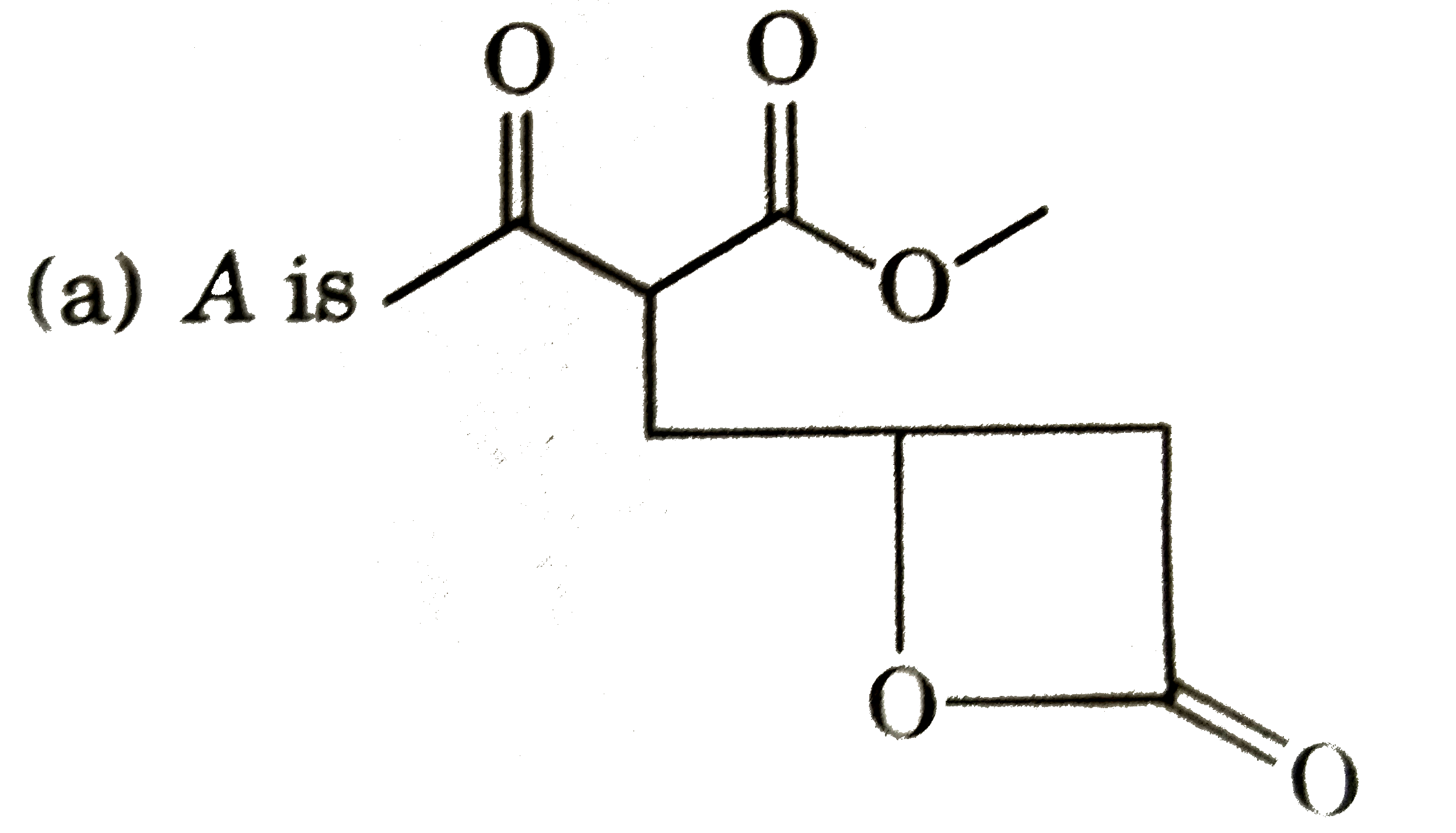 B
B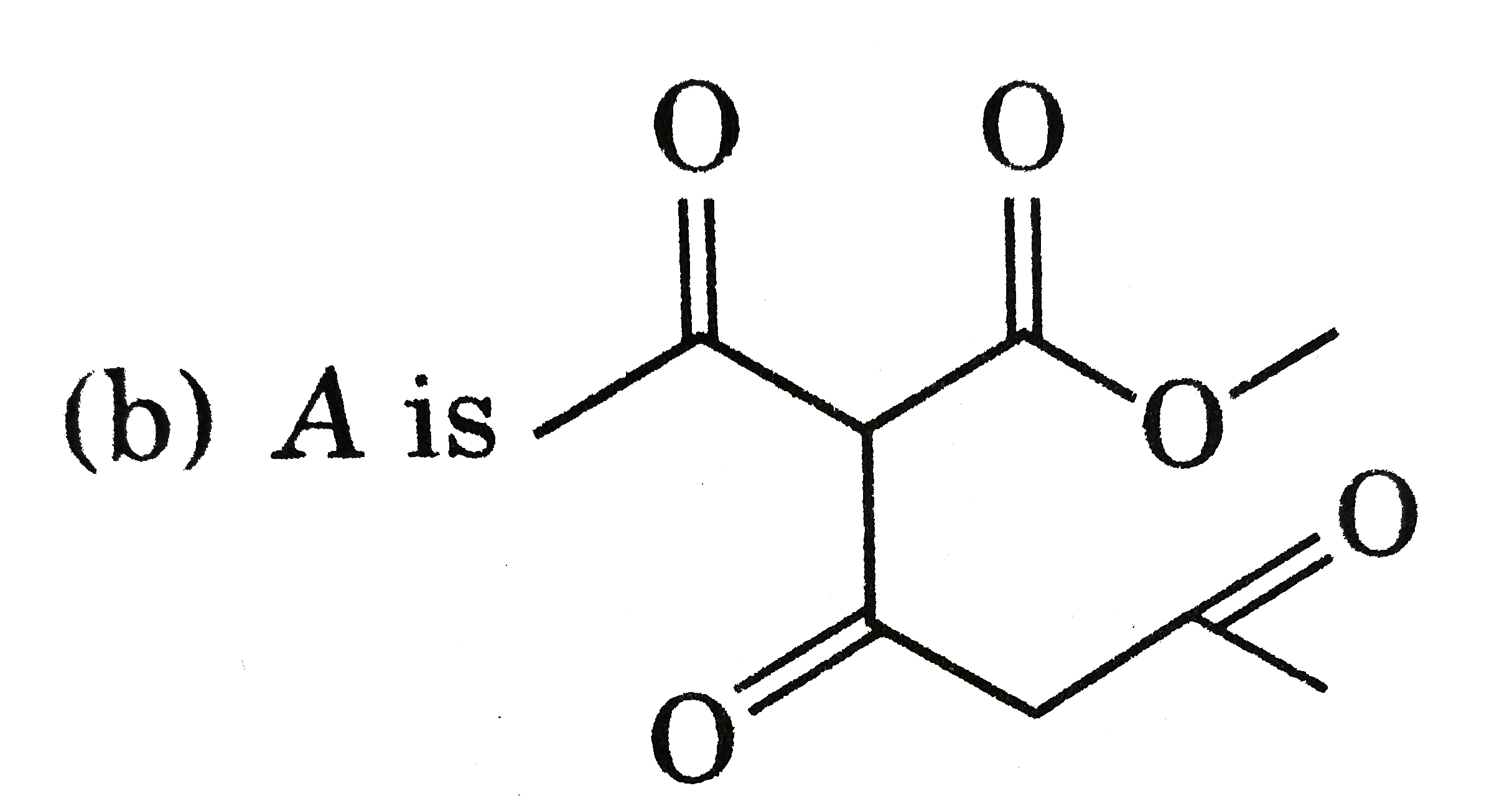 C
C DThis is an example of Michael addition
DThis is an example of Michael addition

