A, B and C are three complexes of chromium (III) with the empirical formula H12O6Cl3Cr. All the three complexes have water and chloride ion as ligands. Complex A does not react with concentrated H2SO4, whereas complexes B and C lose 6.75% and 13.5% of their original weight, respectively, on treatment with concentrated H2SO4.
Complexes A, B and C are not distinguished by:
molar conductivity data
by using P4O10
qualitative test by AgNO3
quantitative test by AgNO3
The correct Answer is:C
Quantitative pptn of AgCl is required.
Topper's Solved these Questions
- COORDINATION COMPOUNDSBOOK - GRB PUBLICATIONCHAPTER - COORDINATION COMPOUNDSEXERCISE - COMPREHENSION 6
2 Videos
- COORDINATION COMPOUNDSBOOK - GRB PUBLICATIONCHAPTER - COORDINATION COMPOUNDSEXERCISE - COMPREHENSION 7
2 Videos
- COORDINATION COMPOUNDSBOOK - GRB PUBLICATIONCHAPTER - COORDINATION COMPOUNDSEXERCISE - COMPREHENSION 4
3 Videos
- CHEMICAL KINETICSBOOK - GRB PUBLICATIONCHAPTER - CHEMICAL KINETICSEXERCISE - Subjective Type
63 Videos
- D-BLOCK ELEMENTSBOOK - GRB PUBLICATIONCHAPTER - D-BLOCK ELEMENTSEXERCISE - Subjective Type
18 Videos
Similar Questions
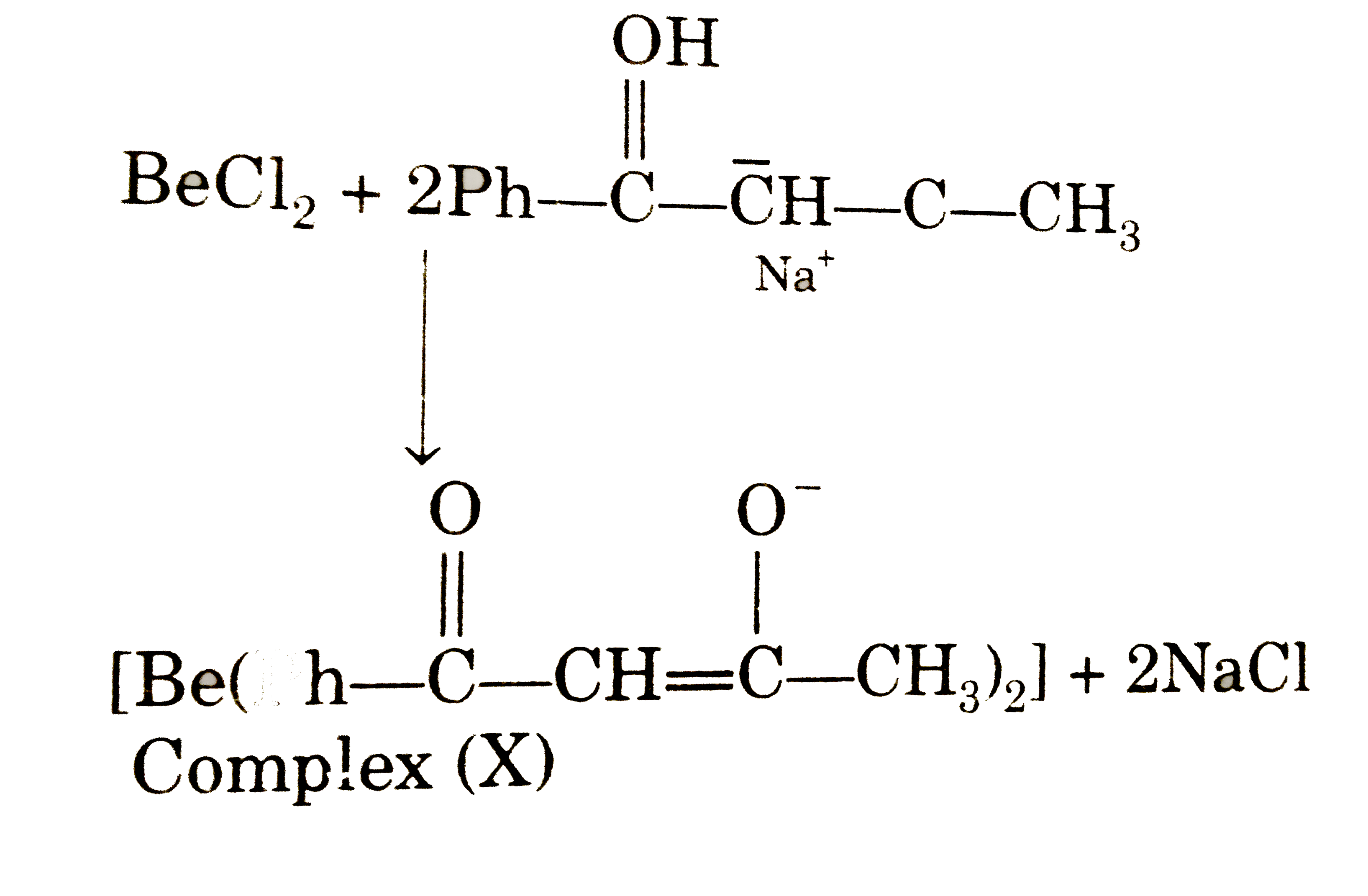
The complex (X) has tetrahedral geometry. The correct name of the complex (X) is :
View SolutionAccording to cystal field theory, interaction between central metal atom/ion and ligand is electrostatic in nature. In free metal ions, the five d-orbitals are degenerate. However, in a ligand field e.g., tetrahedral, octahedral, square planar, square pyramidal, trigonal bipyramidal, the degeneracy of 5d-orbitals is lost.
If lobes of d-orbitals of central metal atom/ion are along the axes through which ligands are approaching, the energy of corresponding d-orbital is raised more than the d-orbitals having lobes between teh axes. e.g., octahedral complexes, square planar complexes, square pyramidal compexes.
Which of the following orbitals has the highest energy in square pyramidal ligand field ?
View SolutionComplex compound are molecular compounds which rectain their identities even when dissolved in water. They do not give all the simple ions in solution but instead furnish complex ions with complicated structures. The complex compounds are often called coordination compounds because certain groups called ligands are attached to the central metal ion by coordinate or dative bonds. Coordination compounds exhibit isomerism, both structural and stereoisomerism. The structure, magnetic property, colour and electrical properties of complexes are explained by various theories.
Arrange the following compounds in order of their Molar conductance :
(P)K[Co(NO2)4(NH3)4(NH3)2]
(Q) [Cr(ONO)3(NH3)3]
(R)[Cr(NO2)(NH3)5]3[Co(NO2)6]2
(S)Mg[Cr(NO2)5(NH3)]
View SolutionCalculate the specific activity of a radioactive substance .25098 Cf if its half life is 6.93 min . Express your answer in terms of 1016 dps.
(Use:NA=6×1023)
View SolutionNumber of halide ions among (F−,Cl−,Br−,I−) which change their oxidation number on heating with MnO2+conc.H2CO4
View SolutionA,B and C are three complexes of chromium(III) with the empirical formula H12O6C13Cr All the three complexes not react with concentrated H2SO4 whereas complexes B and C lose 6.75% and 13.5% of their original mass respectively, on treatment on treatment with conectrated H2SO4 Identify A,B and C .
View SolutionA, B and C are three complexes of chromium (III) with the empirical formula H_(2)O_(6)Cl_(3)Cr . All the three complexes have water and chloride ion as ligands. Complex A does not react with concentrated H_(2)SO_(4) , whereas complexes B and C lose 6.75 % and 13.5 % of their original mass, respectively, on treatment with concentrated H_(2)SO_(4) . Identify A, B and C
View SolutionA, B and C are three complexes of chromium with the empirical formula H_(12)O_(6)Cl_(3)Cr . All the three complexes have Cl and H_(2)O as ligands. Complex A does not react with conc. H_(2)SO_(4) . Complexes B and C lose 6.75% and 13.5% of their original weight respectively on heating with conc. H_(2)SO_(4) . Identify A, B and C.
View SolutionA, B and C are three complexes of chromium (III) with the empirical formula H_(12)O_(6)Cl_(3)Cr . All the three complexes have water and chlorie ions as ligands. Complex A does not react with conc. H_(2)SO_(4) whereas complexes B and C lose 6.75% and 13.5% of their original weight respectively, on treatment with concentrated H_(2)SO_(4) . The complex A is
View SolutionA, B and C are three complexes of chromium (III) with the empirical formula H_(12)O_(6)Cl_(3)Cr . All the three complexes have water and chlorie ions as ligands. Complex A does not react with conc. H_(2)SO_(4) whereas complexes B and C lose 6.75% and 13.5% of their original weight respectively, on treatment with concentrated H_(2)SO_(4) . The complex B is
View SolutionA,B and C are three complexes of chromium(III) with the empirical formula H12O6C13Cr All the three complexes not react with concentrated H2SO4 whereas complexes B and C lose 6.75% and 13.5% of their original mass respectively, on treatment on treatment with conectrated H2SO4 Identify A,B and C .
View SolutionConcentrated H_(2)SO_(4) will not dehydrate the following complex :
View SolutionA, B and C are three complexes of chromium (III) with the empirical formula H12O6Cl3Cr. All the three complexes have water and chloride ion as ligands. Complex A does not react with concentrated H2SO4, whereas complexes B and C lose 6.75% and 13.5% of their original weight, respectively, on treatment with concentrated H2SO4.
According to the Werner theory, the structure of complex A is represented by :
View SolutionA, B and C are three complexes of chromium (III) with the empirical formula H_(12)O_(6)Cl_(3)Cr . All the three complexes have water and chloride ion as ligands. Complex A does not react with concentrated H_(2)SO_(4) , whereas complexes B and C lose 6.75% and 13.5% of their original weight, respectively, on treatment with concentrated H_(2)SO_(4) . Complexes A, B and C are not distinguished by:
View SolutionA, B and C are three complexes of chromium (III) with the empirical formula H_(12)O_(6)Cl_(3)Cr . All the three complexes have water and chloride ion as ligands. Complex A does not react with concentrated H_(2)SO_(4) , whereas complexes B and C lose 6.75% and 13.5% of their original weight, respectively, on treatment with concentrated H_(2)SO_(4) . Which type of isomerism is possible in complex C?
View Solution

