Maximum number of lone pair electrons present in the molecules among the following :
A
XeF2
B
XeF4
C
XeOF4
D
H2O
Text Solution
The correct Answer is:A
|
Similar Questions
Knowledge Check
Numbers of lone pairs present in ozone molecule is :-
A4B5C6D12Which has maximum number of lone pair of electrons present on central atom i.e., xenon?
AXeF4BXeO2F2CXeF2DXeOF4
Number of lone pairs present in water.
View SolutionNumber of lone pairs of electrons in P4 molecule = …….. .
View SolutionThe total number of lone pair of electrons in the given molecule is :
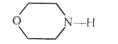
View SolutionWhich has maximum number of lone pair of electrons present on central atom i.e., xenon?
View SolutionWhich has maximum number of lone pair of electrons present on central atom i.e., xenon?
View SolutionThe number of lone pair of electrons in a molecule of ethylene glycol is____
View Solution

