What is the shape of the ClF3 molecule?
Trigonal planar
Trigonal pyramidal
T-shaped
Tetrahedral
The correct Answer is:C
Step by step video, text & image solution for What is the shape of the ClF_(3) molecule? by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- CHEMICAL BONDING-IBook:GRB PUBLICATIONChapter:CHEMICAL BONDING-IExercise:C.Identification of Planes; Allenes
Explore 22 Videos
- CHEMICAL BONDING-IBook:GRB PUBLICATIONChapter:CHEMICAL BONDING-IExercise:D.
Explore 54 Videos
- CHEMICAL BONDING-IBook:GRB PUBLICATIONChapter:CHEMICAL BONDING-IExercise:Subjective Type
Explore 120 Videos
- CARBOXYLIC ACID AND DERIVATIVES, AMINES AND OTHER NITROGEN COMPOUNDSBook:GRB PUBLICATIONChapter:CARBOXYLIC ACID AND DERIVATIVES, AMINES AND OTHER NITROGEN COMPOUNDSExercise:Subjective type
Explore 19 Videos
- CHEMICAL KINETICSBook:GRB PUBLICATIONChapter:CHEMICAL KINETICSExercise:Subjective Type
Explore 63 Videos
Similar Questions
Nodal planes of π-bonds in CH2=C=C=CH2 are located in,
View SolutionConsider the following statements :
(P) Steric number '7' gives sp3d3 hybridisation
(Q) In ClF3, at least one bond angle is exactly 180∘
(R) Lone pair does not cause any distrotion in the bond angle
The above statements P, Q, R respectively are : (T = True, F = False)
View SolutionThe gaseous potassium chloride molecule has a measured dipole moment of 10.0D, which indicates that it is a very polar molecule. The separation between the nuclei in this molecule is 2.67×10−8cm. Calculate the percentage ionic character in KCl molecule.
View SolutionWrite the number of CO2 molecule :
For example if all are releasing one CO2 molecule fill 1111 in OMR sheet
View SolutionCalculate the specific activity of a radioactive substance .25098 Cf if its half life is 6.93 min . Express your answer in terms of 1016 dps.
(Use:NA=6×1023)
View SolutionThe shape of the molecule ClF_(3) is -
View SolutionWhat is the shape ClF3 molecule ?
View Solutionनिम्न अणुओं में संकरण तथा आकृति बताइए ।
(i) ClF3 (ii) SF4
View SolutionThe most stable shape of ClF3 is shown by
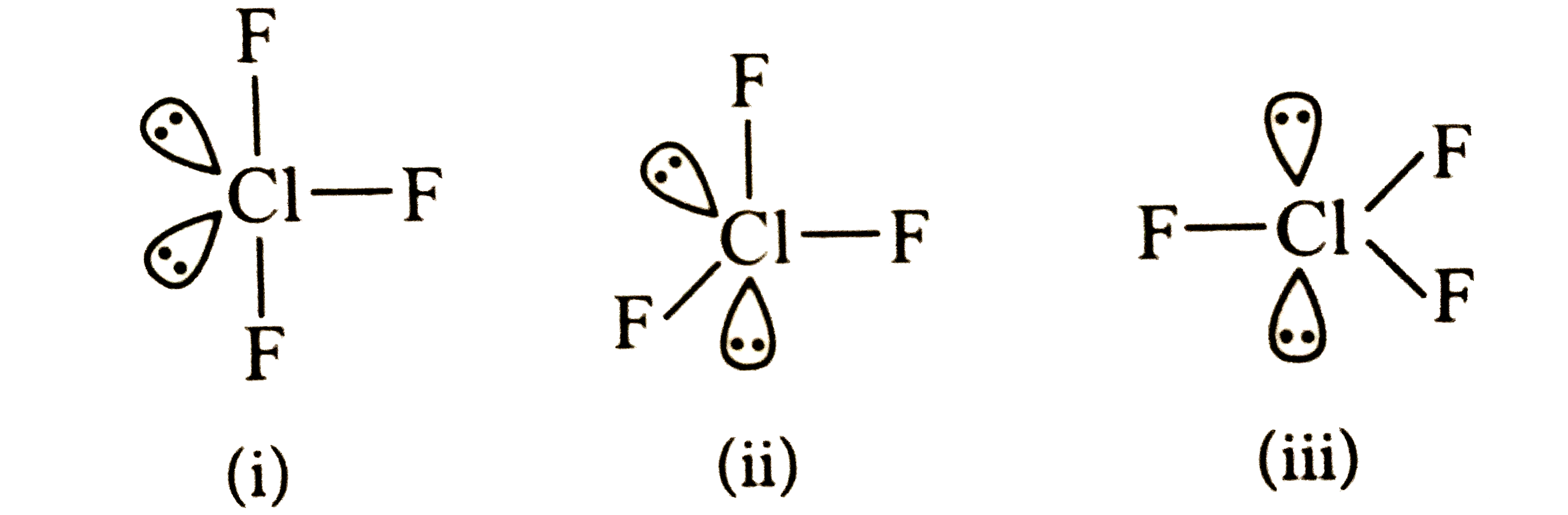
View SolutionShape of ClF3 is
View SolutionWhat is the shape of ClF3,BrF5,IF7 molecules.
View SolutionClF3,BF3एवं NH3अणुओ में समतलीय त्रिकोणीय ज्यामिति वाला अणु है -
View SolutionWhat are the shapes of BrF_(3) , ClF_(5) and IF_(7) molecules ?
View SolutionWhat are the sHapes of ClF3,XeO2F2andBrF5 ?
View SolutionPredict the shape of ClF_(3) according to VSEPR theory.
View Solution
