Find the number of σ bond and lone pair respectively in hydrated borax.
Video Solution
Text Solution
The correct Answer is:34,34
Na2B4O7⋅10H2O

sigma-"bond"rarrunderset(8H_(2)O)ubrace(2xx8)+underset(sp^(3)B)ubrace(4xx2)+underset(sp^(2)B)ubrace(3xx2)+underset(O-H)ubrace(1xx4)=34
l.p.⇒17'O'×2=34
|
Similar Questions
Knowledge Check
The number of σ bonds, π bonds and lone pair
A7σ bonds,2π bonds,2lone pairsee−B6σ bonds,1πbond,4lone paire−C7σbonds,1πbond,4lone paire−DNone of these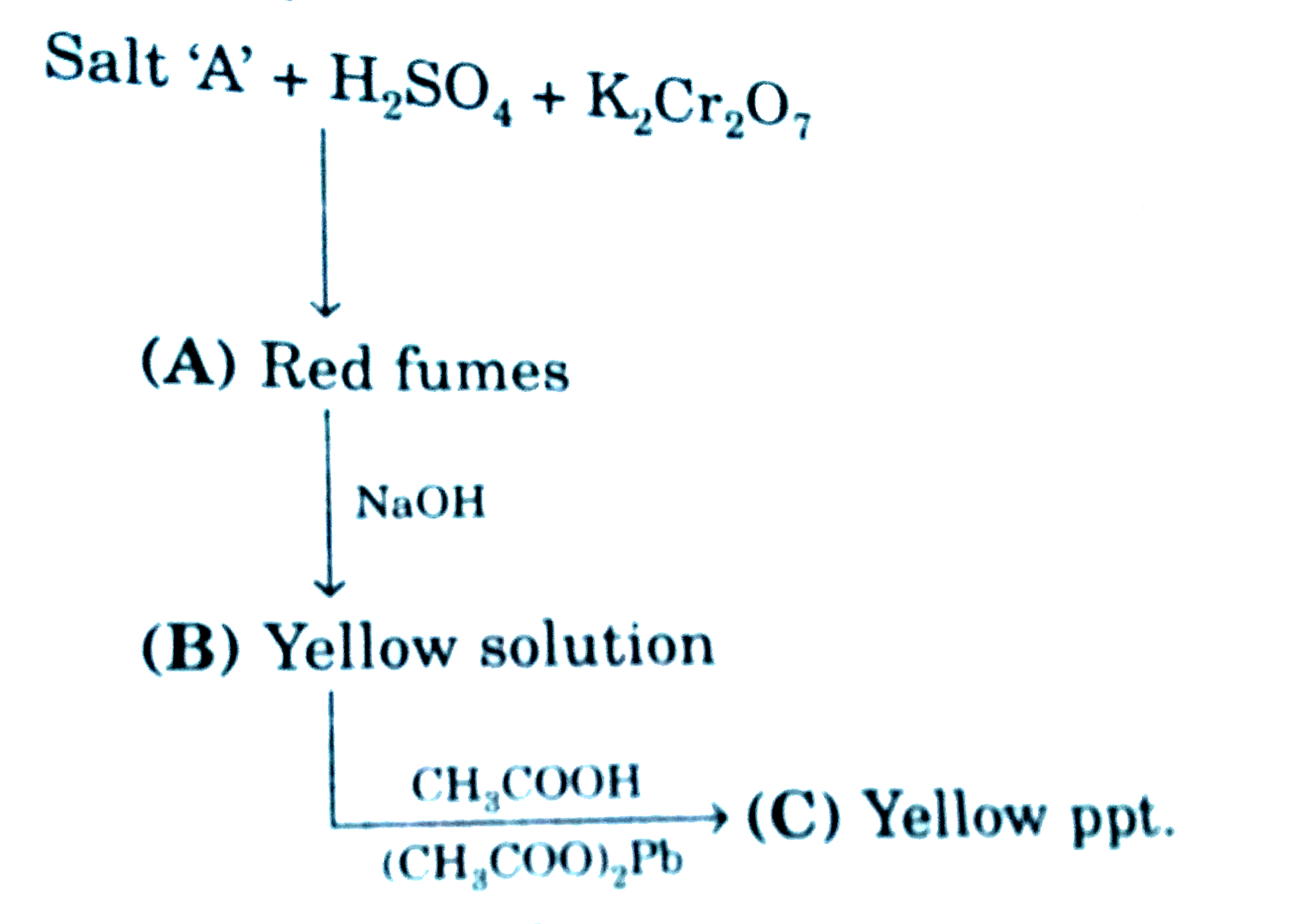
Find the sum of number of σ-bond in A,B and C.A12B4C8D32In the resonating structure of aniline, the number of σ and π bonds are respectively
A12,σ,3πB14σ,3πC6σ,6πD3σ,3π.
The number of σ bonds, pi bonds and lone pair of electrons present in acetic acid are
View SolutionIn NO−3 ion, the number of bond pair and lone pair of electrons on nitrogen atom are:
View SolutionThe number of σ- and π-bond in 5-oxohexanoic acid, respectively, is :
View SolutionIn NO−3 ion, the number of bond pair and lone pair of electrons on nitrogen atom are:
View SolutionThe number of σ- and π-bonds in 5-oxohexanoic acid, respectively, is:
View Solution
