Derive the equation for 'Wrev' in isothermal reversible process.Solution in Telugu
Work done during the expansion of a gas against external pressure can be expressed as
W=Pext(−ΔV)=−Pext(Vf−Vi)
In isothermal reversible process the above equation can be written as
Wrev=−Vf∫ViPextdV=Vf∫Vi(Pin±dP)dV
Since dp×dv is very small it can be neglected and we can write the equation as
Wrev=ViPVfindV
Now the pressure of the gas Pin can be written as P.
This can be obtained from ideal gas equation.
P=nRTv
Therefore at constant temperature (isothermal process)
Wrev=Vi∫VfnRTdVV=−nRTInVfVi
Wrev=−2.303nRTlogVfVi
Topper's Solved these Questions
Similar Questions
What are the postulates of Bohr's model of hydrogen atom?
View SolutionDerive the equation for work done in case of isothermal process.
View SolutionReversible Isothermal and Adiabatic process
View SolutionReversible Isothermal and Adiabatic process
View SolutionIf w_(1), w_(2), w_(3) and w_(4) for an ideal gas are magnitude of work done in isothermal, adiabatic, isobaric and isochoric reversible expansion processes, the correct order will be :
View SolutionIsothermal Reversible Process and Isothermal irreversible Process || Various important Points || Comparison OF P-V Graph for Isothermal Reversible Process and Isothermal irreversible Process
View SolutionIf the system involves gaseous substance, then pressure volume work Wpv=−PextΔV. It has been observed that,
Wrev>Wir.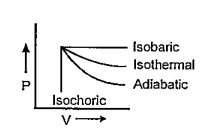
Also the work of isothermal & adiabatic processes are different from each other :
Wisothermalreversib≤=2.303nRTlog(v2v1)
Wadiabaticreversib≤=−Cv(T2−T1)
If W1,W2,W3,W4 are work done in isothermal, adiabatic, isobaric & isochoric reversible processes, then correct sequence would be
View SolutionIf the system involves gaseous substance, then pressure volume work W_(pv) = -P_(ext)DeltaV . It has been observed that, W_(rev) gt W_(ir) . Also the work of isothermal & adiabatic processes are different from each other : W_(isothermal reversible) = 2.303 nRT log(v_2/v_1) W_(adiabatic reversible) = -C_v(T_2-T_1) P-V plot for two gases during adiabatic processes are given as follows : Curves A & B should correspond to respectively
View Solution
