The sign and magnitude of Gibbs energy change of a chemical process tells about its spontaneity and useful work that could be extracted from it. Explain.Solution in Telugu
The process which takes place on its own accord without the aid of an external agency is called spontaneous process. To sum up, the criteria for spontaneous of process in terms of ΔG can be given as
i) If ΔG is -ve the process is spontaneous
ii) If ΔG is zero, the process does not occur (or) the system is in equilibrium
iii) If ΔG is +ve, the process does not occur in the forward direction The spontaneity of a reaction and the algebraic signs of ΔH,ΔS,ΔG and the magnitude of T are related to each others as given in the below table.
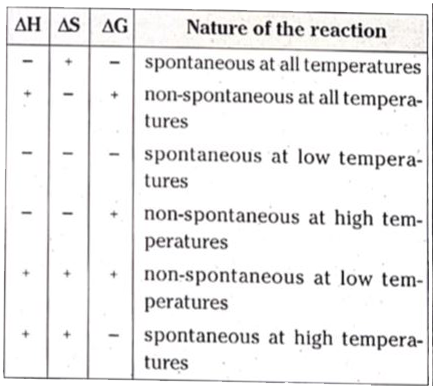
For a spontaneous process in an isolated system the change in entropy should be positive. But many reactions with ΔS in negative are known to occur spontaneously. These reactions takes place on account of coupling of these reactions with reactions of large negative ΔG values.
Topper's Solved these Questions
Similar Questions
What are the postulates of Bohr's model of hydrogen atom?
View Solution(i) Spontaneity of a process can be determined from the entropy change and also from the Gibbs free energy change. But the Gibbs free energy change in more useful than entropy change to determine the spontaneity of a process. Explain it.
(ii) Show that Gibbs energy change of a system at certain temperature and pressure, ΔG=ΔH−TΔS.
View Solution................... Is a process in which electrical energy is used to cause a non-spontaneous chemical reaction.
View SolutionDescribe Gibbs energy and spontaneity.
View SolutionThe spontaneity of a process is expressed in terms of a change in Gibbs energy.
a) What is meant by a change in Gibbs energy of a system?
b) How is it related to the enthalpy and entropy of a system?
c) How is it useful in predicting the feasibility of a process?
View Solutiongibbs free energy,condition for spontaneity,physical significance OF gibbs free energy,gibbs free energy OF chemical reaction
View SolutionAssertion (A): The thermodynamic factor which determines the spontaneity of a process is the free energy. For a process to be spontaneous the free energy must be-ve.
Reason (R ) : The change in free energy is related to the change in a process must always be positive if its is spontaneous.
View SolutionExplain spontaneity of a process in terms of Gibbs energy.
View Solution

