The arrow mark between the products and reactants of a chemical equation shows ………………of the reaction. Solution in Telugu
direction
Similar Questions
Write the balanced chemical equation for the reaction between sodium carbonate and hydrochlorc acid indicating the physical states of reactants and products.
View SolutionIn a chemical reaction if all reactants and products are in liquid state then:
View SolutionAfter a chemical reaction the total mass of reactant and products
View SolutionAn arrow pointing downwards in a chemical reaction shows……
View Solution(i) The reactions are written on the left side of the chemical equation (ii) The products are written on the right side of the equation (iii) Arrow marks are placed between the reducer and the product
View SolutionWrite a balanced chemical equation for the reaction between sodium carbonate and hydrochloric acid indicating the physical state of the reactants and the products.
View SolutionComplete the statement by filling the gaps using appropriate term from the terms given in the bracket : ( slow, coloured, arrow,fast,smell , milky , physical, product , chemical , reactant , covalent, ionic, octet , duplet, exchange , sharing , equality sign) An …………. Is drawn in between the reactants and products while writing the equation for a chemical reaction .
View SolutionCase 1: A chemical equation is the representation of chemical change in terms of symbols and formulae of reactants and products. The substances which react are written on the left hand side of the arrow and are termed as reactants while the substances produced as result of reaction are called products and are written on the right hand side of the arrow. The arrowhead shows the direction of the reaction. A chemical equation in the whcih the number of atoms of each element on reactant side is equal to that on the product side is said to be balanced.
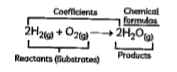
Which of the following statement is incorrect regarding th significance of the chemical equations?
View Solution