The root mean square speed of hydrogen molecule at 300 K is 1930m/s. Then the root mean square speed of oxygen molecules at 900K will be………..
The correct Answer is:C
Step by step video, text & image solution for The root mean square speed of hydrogen molecule at 300 K is 1930m/s. Then the root mean square speed of oxygen molecules at 900K will be……….. by Physics experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
- KINETIC THEORY OF GASESBook:CENGAGE PHYSICSChapter:KINETIC THEORY OF GASESExercise:Multiple correct
Explore 3 Videos
- KINETIC THEORY OF GASESBook:CENGAGE PHYSICSChapter:KINETIC THEORY OF GASESExercise:Compression
Explore 2 Videos
- KINETIC THEORY OF GASESBook:CENGAGE PHYSICSChapter:KINETIC THEORY OF GASESExercise:Comprehension Type
Explore 5 Videos
- KINEMATICS-2Book:CENGAGE PHYSICSChapter:KINEMATICS-2Exercise:Exercise Integer
Explore 9 Videos
- KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSBook:CENGAGE PHYSICSChapter:KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSExercise:Interger
Explore 11 Videos
Similar Questions
A steel tank contains 300 g of ammonia gas (NH3) at a pressure of 1.35×106 Pa and a temperature of 77∘C . Later the temperature is 22∘C and the pressure is 8.7×105 Pa .
View SolutionOne mole of an ideal monatomic gas undergoes a linear process from A to B , in which is pressure P and its volume V change as shown in figure .
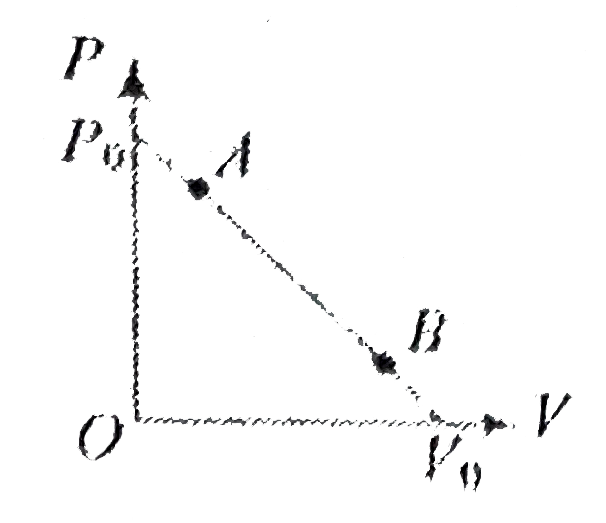
The absolute temperature T versus volume V for the given process is
View SolutionSuppose there are N molecules each of mass m, of an ideal gas in a container. The x component of velocity of a molecule is denoted by ux. The gas is enclosed using a horizontal piston of area A as shown.

The pressure of the gas is
View SolutionA particle is projected with velocity u at angle θ with horizontal. Find the time when velocity vector is perpendicular to initial velocity vector.
View Solution8g of oxygen, 14 g of nitrogen and 22 g carbon dioxide are mixed in an encloser of volume 10 litre and temperature 27∘C. Calculate the pressure exerted by the mixture , R=8.3Jmole−1K−1, Molecular weight of oxygen , nitrogen and carbon 32 , 28 and 44 respectively.
View SolutionCalucate the root mean square speed of hydrogen molecules at 373.15K.
View Solutionकमरें के ताप पर हाइड्रोजन के अणुओं की वर्ग-माध्य-मूल चाल तथा ऑक्सीजन के अणुओं की वर्ग-माध्य-मूल चाल का अनुपात होगा:
View Solutionकिसी ताप पर ऑक्सीजन के अणुओं की वर्ग-माध्य-मूल चाल 150 मीटर/सेकण्ड है। उसी ताप पर हाइड्रोजन के अणुओं की वर्ग-माध्य-मूल चाल की गणना कीजिए। ऑकसीजन तथा हाइड्रोजन के अणुभार क्रमशः 32 तथा 2 है।
View SolutionAt what temperature will be the oxygen molecules have the same root mean square root mean square speed as hydrogen molecules at 300 K ?
View Solutionवह ताप, जिस पर हीलियम गैस के अणुओं की वर्ग माध्य मूल चाल 0∘C पर हाइड्रोजन गैस के अणुओं की वर्ग माध्य मूल चाल के बराबर होगी है :
View Solutionवह ताप, जिस पर हाइड्रोजन गैस के अणुओं की वर्ग माध्य मूल चाल, ऑक्सीजन गैस के अणुओं की 47∘C पर वर्ग माध्य मूल चाल के समान होगी है :
View SolutionAt what temperature will oxygen molecules have the same root mean square speed as hydrogen molecules at 300 K ?
View Solution300 K पर हाइड्रोजन के अणुओं का वर्ग माध्य मूल वेग 1930 मी./से. है। 900 K पर ऑक्सीजन के अणुओं का वर्ग माध्य मूल वेग होगा
View Solutionयदि हाइड्रोजन अणुओं की वर्ग माध्य मूल चाल 47∘Cपर ऑक्सीजन अणुओं की वर्ग माध्य मूल चाल के बराबर है तो हाइड्रोजन का ताप है
View SolutionThe root mean square soeed of hydrogen molecules at room temperature is 2400 ms^(-1) . At room temperature the root mean square speed of oxygen molecules would be :
View Solution
