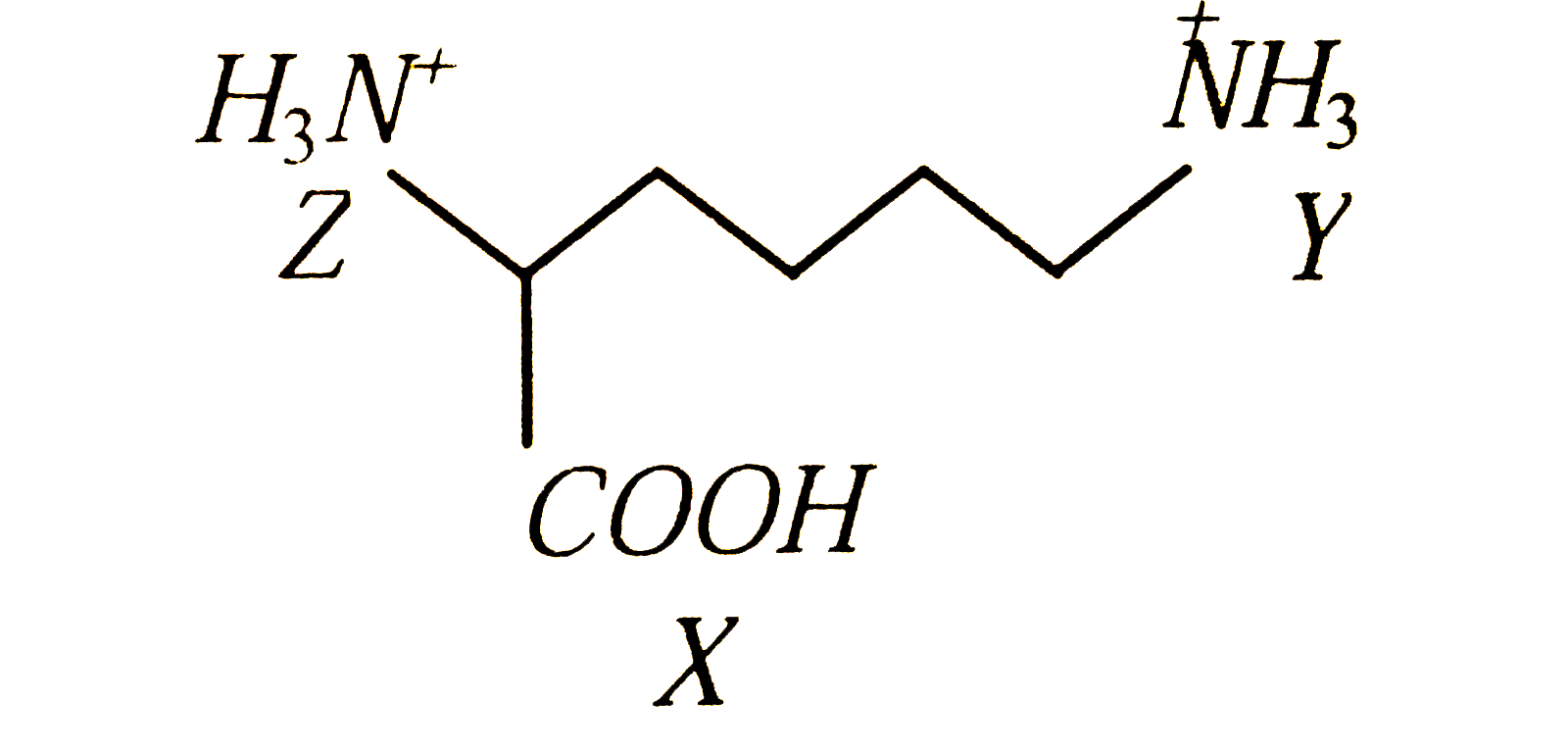when the substance below are arranged in order oif increasing entropy value, S∘,at25∘C which is the correct order?
A
CO2(s)<CO2(aq)<CO2(g)
B
CO2(g)<CO2(aq)<CO2(s)
C
CO2(s)<CO2(g)<CO2(aq)
D
CO2(g)<CO2(s)<CO2(aq)
Video Solution
Text Solution
The correct Answer is:A
|
Answer
Step by step video, text & image solution for when the substance below are arranged in order oif increasing entropy value, S^(@), at 25^(@)C which is the correct order? by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Similar Questions
Knowledge Check
Arrange in order of increasing I.P.
AMg<Al<Si<PBAl<Si<P<MgCSi<P<Mg<AlDAl<Mg<Si<P
Arrange in order of increasing acid strengthAX>Z>YBZ<X>YCX>Y>ZDZ>X>Y
Arrange in order of increasing acid strengthAX>Z>YBZ<X>YCX>Y>ZDZ>X>Y
Arrange the following in the order of increasing entropy:
View SolutionWhich of the following is arranged in order of increasing density
View SolutionIn which list are the ions arranged in order of increasing size?
View SolutionWhich of the following is arranged in order of increasing density?
View SolutionThe ions which are arranged in correct order of increasing radii are
View Solution


_CHE_E06_190_Q01.png)