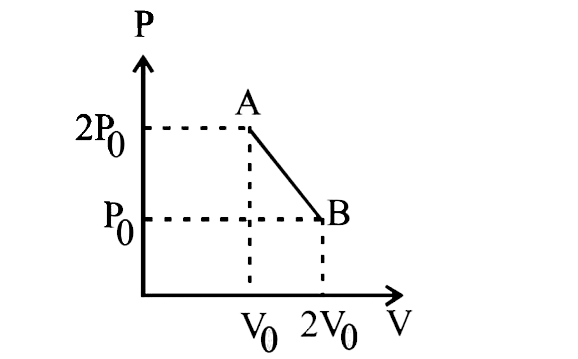
One mole of ideal gas undergoes a linear process as shown in figure below. Its temperature expressed as function of volume V is -
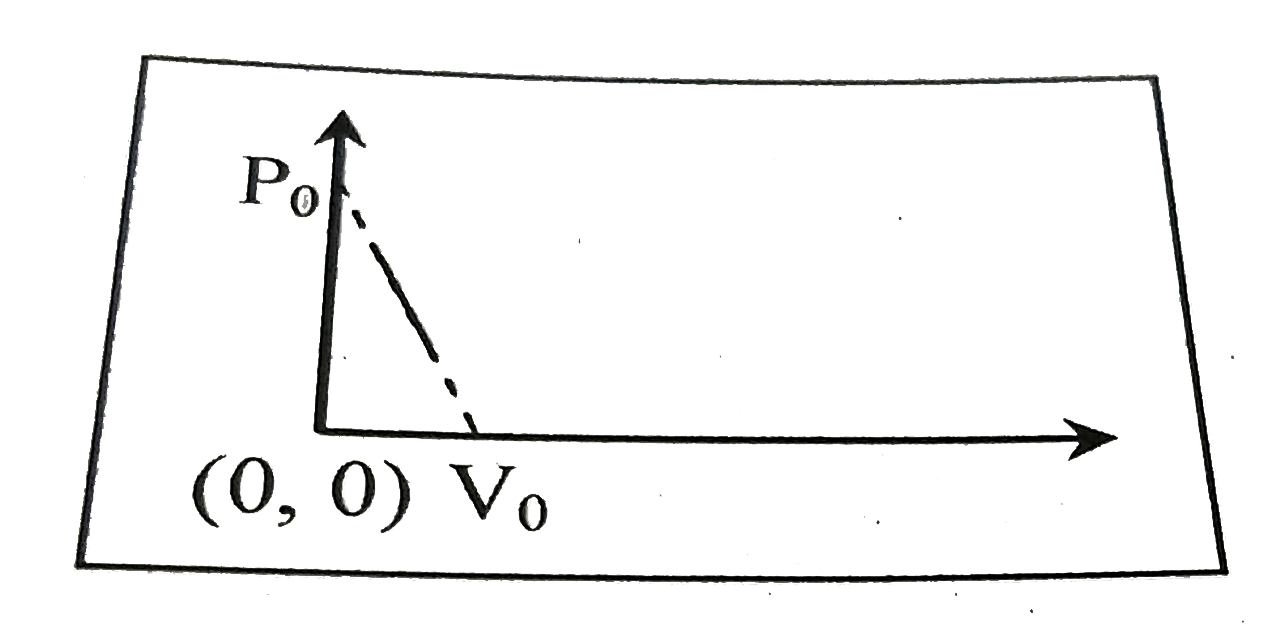
P0V0R
P0VR
P0VR(1−VV0)
P0V0R(1−(VV0)2)
The correct Answer is:C
Step by step video, text & image solution for One mole of ideal gas undergoes a linear process as shown in figure below. Its temperature expressed as function of volume V is - by Physics experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
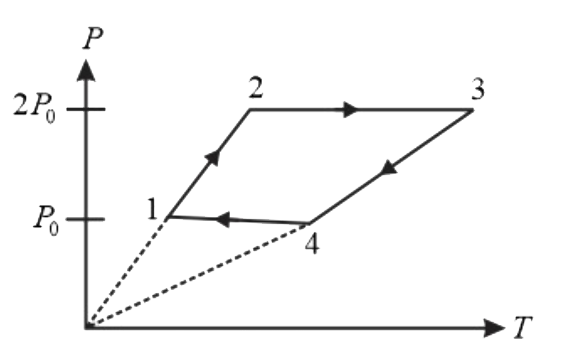
One mole of an ideal monoatomic gas undergoes a cyclic process, as shown in the figure. If the temperature of the gas at state 1 is 300 K and at state 4 is 500 K, then heat exchanged during process 2→3, is
View SolutionOne mole of monoatomic ideal gas undergoes a process ABC as shown in figure. The maximum temperature of the gas during the process ABC is in the form xPVR . Find x .
_E01_281_Q01.png)
View Solution
Knowledge Check
An ideal gas undergoes the cyclic process shown in a graph below :
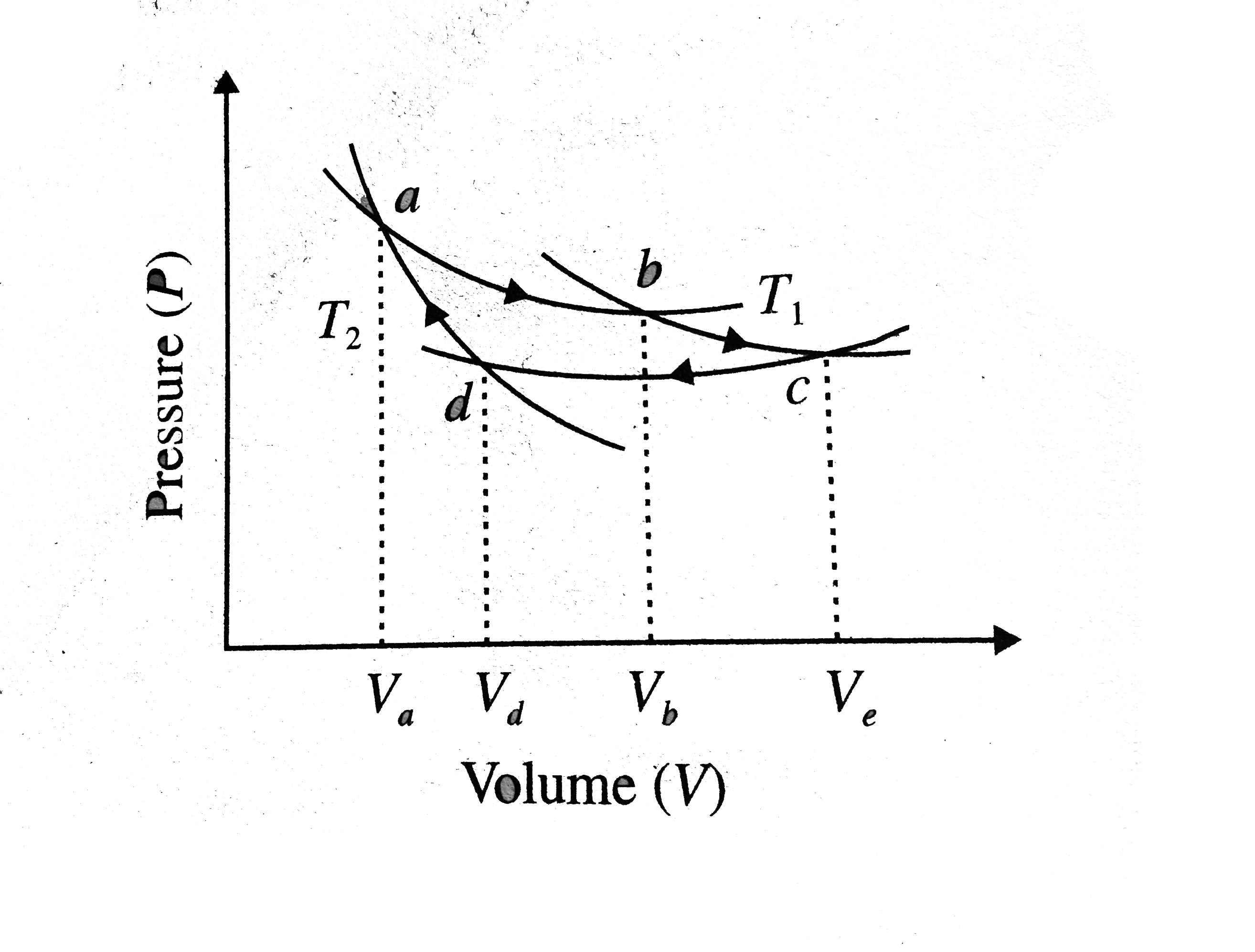 AT1=T2BT1>T2CVaVc=VbVdDVaVb=VcVd
AT1=T2BT1>T2CVaVc=VbVdDVaVb=VcVdOne mole of an ideal monoatomic gas undergoes a cyclic process, as shown in the figure. If the temperature of the gas at state 1 is 300 K and at state 4 is 500 K, then heat exchanged during process 2→3, is
 A1000 RB600 RC750 RD800 R
A1000 RB600 RC750 RD800 ROne mole of an ideal monoatomic gas undergoes a cyclic process as shown in figure . Temperature at point 1 =300K and process 2−3 is isothermal .
_S01_113_O01.png) .
.
Net work done by gas in complete cycle isA(9ln3+12)P0V0B(9ln3+4)P0V0C(9ln3−4)P0V0D(9ln3−8)P0V0
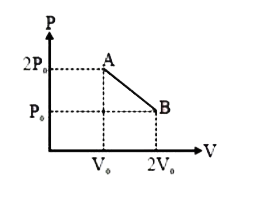
n' moles of an ideal gas undergoes a process A→B as shown in the figure. The maximum temperature of the gas during the process will is p0v04nR then value of N is:
View SolutionOne mole of an ideal monoatomic gas undergoes a cyclic process as shown in figure . Temperature at point 1 =300K and process 2−3 is isothermal .
_S01_114_O01.png)
Heat capacity of process 1→2 is
View SolutionOne mole of an ideal monoatomic gas undergoes a cyclic process as shown in figure . Temperature at point 1 =300K and process 2−3 is isothermal .
_S01_115_O01.png)
The efficiency of cycle is
View Solutionn' moles of an ideal gas undergoes a process A→B as shown in the figure. The maximum temperature of the gas during the process will be:
View SolutionOne mole of ideal gas undergoes a cyclic process ACBA as shown in figure. Process AC is adiabatic. The temperatures at 1, B and C are 300 K,600 K and 450 K respectively. Choose the correct statement.
View Solution

