Using the data given below:
E∘Cr2O2−7∣Cr3+=1.33VE∘Cl2∣Cl−=1.36V
E∘MnO−4∣Mn2+=1.51VECr3+∣Cr=−0.74V
Find the most stable ion in its reduced forms
Cl−
Cr3+
Cr
Mn2+
The correct Answer is:D
Step by step video, text & image solution for Using the data given below: E_(Cr_(2)O_(7)^(2-)|Cr^(3+))^(@)=1.33V E_(Cl_(2)|Cl^(-))^(@)=1.36V E_(MnO_(4)^(-)|Mn^(2+))^(@)=1.51V E_(Cr^(3+)|Cr)=-0.74V Find the most stable ion in its reduced forms by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- ELECTROCHEMISTYBook:GRB PUBLICATIONChapter:ELECTROCHEMISTYExercise:Match the column type
Explore 16 Videos
- ELECTROCHEMISTYBook:GRB PUBLICATIONChapter:ELECTROCHEMISTYExercise:Subjective type
Explore 39 Videos
- ELECTROCHEMISTYBook:GRB PUBLICATIONChapter:ELECTROCHEMISTYExercise:Multiple objective type
Explore 33 Videos
- D-BLOCK ELEMENTSBook:GRB PUBLICATIONChapter:D-BLOCK ELEMENTSExercise:Subjective Type
Explore 18 Videos
- ENVIRONMENTAL CHEMISTRY Book:GRB PUBLICATIONChapter:ENVIRONMENTAL CHEMISTRY Exercise:Straight objective type
Explore 40 Videos
Similar Questions
Mathc the following columns :
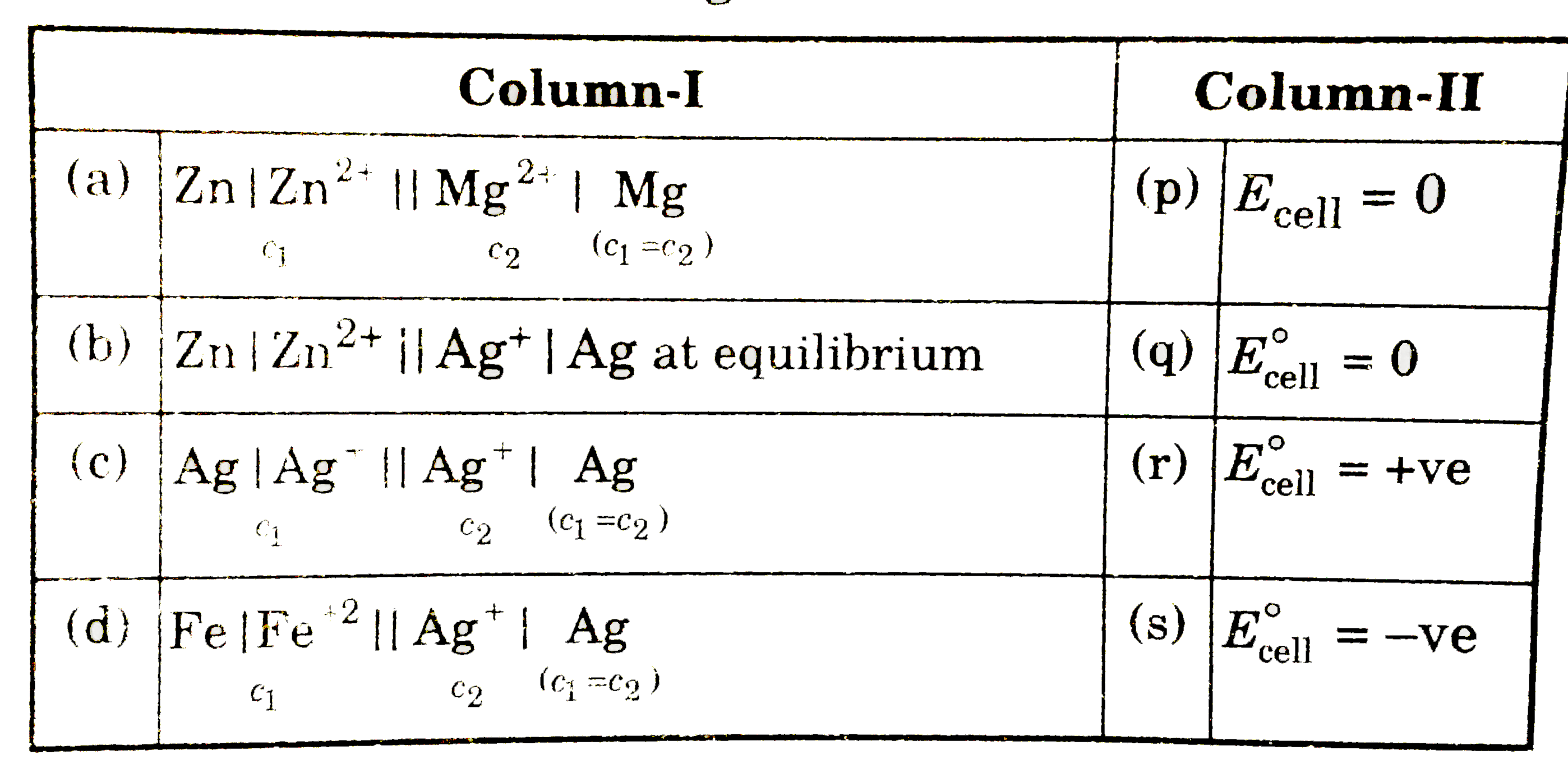
View SolutionThe conductivity ofa solution may be taken to be directly proportional to the total concentration of the charge carriers (ions) present in it in many cases. Using the above, find the percent decrease in conductivity (k) of a solution of a week monoacidic base BOH when its 0.1 M solution is diluted to double its original volume. (Kb=10−5forBOH)(takesqrt(50) = 7.07) (mark the answer to nearest integer)
View SolutionWhich of the following statements is wrong about galvanic cells?
View SolutionNumber of halide ions among (F−,Cl−,Br−,I−) which change their oxidation number on heating with MnO2+conc.H2CO4
View SolutionWhich of the following cannot be degraded by natural processes ?
(P) DDT , (Q) Plastic materials
(R) Heavy metals , (S) Nuclear wastes
View SolutionUsing the data given below:
E∘Cr2O2−7∣Cr3+=1.33VE∘Cl2∣Cl−=1.36V
E∘MnO−4∣Mn2+=1.51VECr3+∣Cr=−0.74V
Mark the strongest reducing agent.
View SolutionUsing the data given below: E_(Cr_(2)O_(7)^(2-)|Cr^(3+))^(@)=1.33V E_(Cl_(2)|Cl^(-))^(@)=1.36V E_(MnO_(4)^(-)|Mn^(2+))^(@)=1.51V E_(Cr^(3+)|Cr)=-0.74V Mark the strongest oxidising agent.
View SolutionUsing the data given below:
E∘Cr2O2−7∣Cr3+=1.33VE∘Cl2∣Cl−=1.36V
E∘MnO−4∣Mn2+=1.51VECr3+∣Cr=−0.74V
In which option the order of reducing power is correct?
View SolutionUsing the data given below: E_(Cr_(2)O_(7)^(2-)|Cr^(3+))^(@)=1.33V E_(Cl_(2)|Cl^(-))^(@)=1.36V E_(MnO_(4)^(-)|Mn^(2+))^(@)=1.51V E_(Cr^(3+)|Cr)=-0.74V Find the most stable oxidised species.
View SolutionUsing the data given below find out the strongest reducing agent. E_(Cr_(2)O_(7)^(2-)//Cr^(3+))^(Theta)=1.33V,E_(Cl_(2)//Cl^(-))^(Theta)=1.36V E_(MnO_(4)^(-)//Mn^(2+))^(Theta)=1.51V,E_(Cr^(3+)//Cr)^(Theta)=-0.74V
View SolutionUse the given data - E_(Cr_(2)O_(7)^(2-)|Cr^(3+))^(@)=+1.33V, E_(Cl_(2)|Cl^(-))^(@)=+1.36V E_(MnO_(4)^(-)|Mn^(2+))^(@)=+1.51V, E_(Cr^(3+)|Cr)^(@)=-0.74V and find out which of the following is the strongest oxidising agent -
View SolutionUse the given data E_(Cr_(2)O_(7)^(2-)|Cr^(3+))^(@)=+1.33V, E_(Cl_(2)|Cl^(-))^(@)=+1.36V E_(MnO_(4)^(-)|Mn^(2+))^(@)=+1.51V, E_(Cr^(3+)|Cr)^(@)=-0.74V find out in which option the order of reducing power is correct -
View SolutionUse the given data - E_(Cr_(2)O_(7)^(2-)|Cr^(3+))^(@)=+1.33V, E_(Cl_(2)|Cl^(-))^(@)=+1.36V E_(MnO_(4)^(-)|Mn^(2+))^(@)=+1.51V, E_(Cr^(3+)|Cr)^(@)=-0.74V and find out the most stable ion in its reduced form -
View SolutionUse the given data -
E∘Cr2O2−7∣Cr3+=+1.33V,E∘Cl2∣Cl−=+1.36V
E∘MnO−4∣Mn2+=+1.51V,E∘Cr3+∣Cr=−0.74Vand find out the most stable oxidised species -
View SolutionUsing the data given below find out the strongest reducing agent E_(Cr_(2)O_(7)^(2-))^(@)Cr^(3+)=1.33V," "E_(Cl_(2)//Cl^(-))^(@)=1.36V E_(MnO_(4)^(-))^(@)//Mn^(2+)=1.51V, E_(Cr^(3+)//Cr)^(@)=-0.74V
View Solution
