A system undergoes a cyclic process in which it absorbs Q1 heat and gives out Q2 heat. The efficiency of the process is η and work done is W. Select correct statement:
W=Q1−Q2
η=WQ1
η=Q2Q1
η=1−Q2Q1
Step by step video & image solution for A system undergoes a cyclic process in which it absorbs Q_(1) heat and gives out Q_(2) heat. The efficiency of the process is eta and work done is W. Select correct statement: by Physics experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
A heat engine undergoes a process in which its intemal energy decreases by 400 J and it gives out 150 J of heat. During the process
View SolutionA system works in a cyclic process. It absorbs 20 calories of heat and rejects 60J of heat during the process. The magnitude of work done (J) is [1calorie=4.2J]:
View Solution
Knowledge Check
A system undergoes a cyclic process in which it absorbs Q, heat and gives out Q2. heat. The efficiency of the process is n and the work done is W.
AW=Q1−Q2Bη=WQ1Cη=Q2Q1Dη=1−Q2Q1A system undergoes a cyclic process in which it absorbs heat Q1 and gives away heat Q2. If the efficiency of the process is η and the work done is W, then
AW=Q1−Q2Bη=WQ1Cη=Q2Q1Dη=1−Q2Q1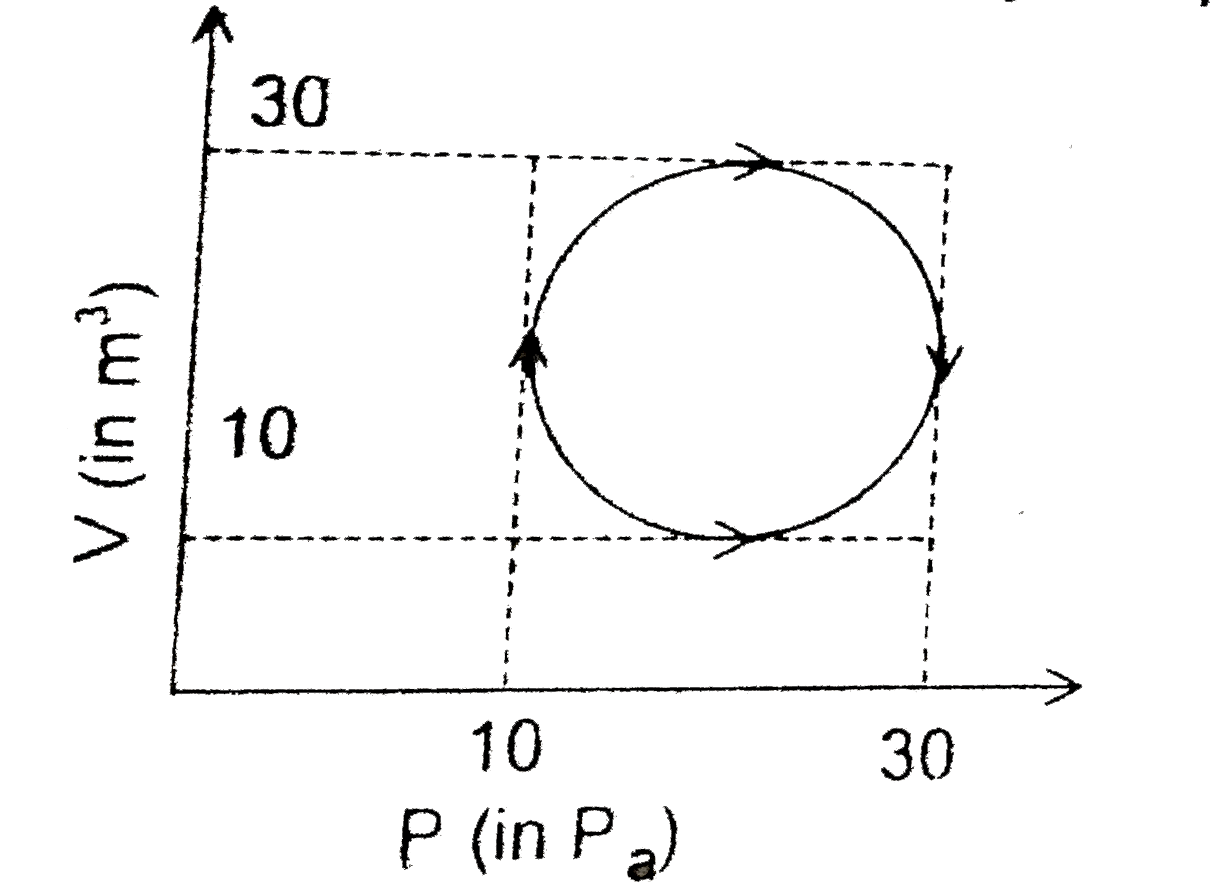
A system works under cyclic process as follow
Heat absorbed during the process isA227×102JB227×103JC227×104JD227×105J
In a cyclic process, work done by the system is
View SolutionIn a cyclic process, work done by the system is
View SolutionA system absorbs 640 J heat and does work. The change in internal energy (ΔU) for the process is 380 J. The work done by the system is __ J
View SolutionA system absorbs 920 J of heat and does work. The change in internal energy (ΔU) for the process is 460 J. The work done by the system is
View SolutionA closed system undergoes a process 1→2 for which the values W1−2 and Q1−2 are 50 kJ and -20 kJ respectively. If the system is returned to state 1 and Q2→1 is + 10 kJ the work done W2→1 is:
View Solution
